A zymogen also called a proenzyme is an inactive precursor of an enzyme.
A zymogen requires a biochemical change for it to become an active enzyme.
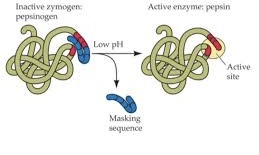
A zymogen requires a biochemical change: hydrolysis reaction revealing the active site, or changing the configuration to reveal the active site, for it to become an active enzyme.
The biochemical change usually occurs in Golgi bodies, where a specific part of the precursor enzyme is cleaved in order to activate it.
The inactivating piece which is cleaved off can be a peptide unit, or can be independently-folding domains comprising more than 100 residues.
These N-terminal extensions of the enzyme or a “prosegment” often aid in the stabilization and folding of the enzyme they inhibit.
The pancreas secretes zymogens partly to prevent the enzymes from digesting proteins in the cells in which they are synthesized.
Enzymes like pepsin are created in the form of pepsinogen, an inactive zymogen.
Pepsinogen is activated when chief cells release it into the gastric acid, whose hydrochloric acid partially activates it.
Accidental activation of zymogens can occur when the secretion duct in the pancreas is blocked by a gallstone, resulting in acute pancreatitis.
In the duodenum, the pancreatic zymogens, trypsinogen, chymotrypsinogen, proelastase and procarboxypeptidase, are converted into active enzymes by enteropeptidase and trypsin.
Examples of zymogens:
Trypsinogen
Chymotrypsinogen
Pepsinogen
Most proteins in the coagulation system (examples, prothrombin, or plasminogen)
Some of the proteins of the complement system
Procaspases
Pacifastin
Proelastase
Prolipase
Procarboxypolypeptidases
