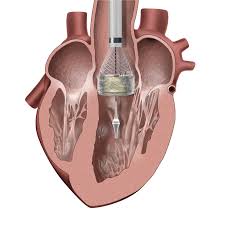
 TAVR uses the transfemoral approach for the treatment of severe, symptomatic aortic stenosis in patients who are inoperable.
TAVR uses the transfemoral approach for the treatment of severe, symptomatic aortic stenosis in patients who are inoperable.
Symptomatic severe aortic stenosis has a two-year mortality rate of nearly 50%.
Uses a TAVR medical device.
Employs an aortic prosthesis either an self-expandable or balloon-expandable system.
Use improves survival as compared with medical therapy in patients with severe aortic stenosis who cannot undergo surgery.
Aortic valve replacement is indicated for patients with severe aortic stenosis with aortic valve area less than 1 cm², who have symptoms, have left ventricle dysfunction, or undergoing cardiac surgery for another indication.
Nearly 30% of patients with severe symptomatic aortic stenosis are not candidates for surgical aortic valve replacement because of multiple medical comorbidities, advanced age, prior surgery, or high risk anatomic features.
Balloon expandable TAVR and surgical aortic valve replacement have similar survival rates at one year among patients considered to be high surgical risk, although the frequency of neurologic events is higher among patients treated with balloon expandable TAVR than among those treated surgically.
Randomized clinical trials involving patients who have less than a 3% risk of surgical operative mortality suggest that TAVR might be the preferred treatment strategy for this particular patient group for aortic stenosis.
The use of a renin-angiotensin system inhibitors are associated with lower mortality and risk for heart failure readmission.
Among patients undergoing TAVR device implantation success is achieved in 92% of cases with an overall in-hospital mortality of 5.5%, and a stroke rate of 2% (Mack, MJ et al).
The rate of 30 day stroke over the first 5 years post TAVR is 2.3%.
Presently only 2 valves are approved in the United States: 1, device a balloon expandable (BE) valve made of bovine pericardium mounted in a cylindrical, short stent and 2,self-expanding (SE) valve made of porcine pericardium mounted in the tall or, nitinol stent with an adaptive shape.
The BE valve is also approved for patients with aortic stenosis or operable but are at high risk for surgery.
Studies of the above valves have shown 30 day outcomes ranging from 5%-9.6% for mortality, 1.5%-4.3% for stroke, 0.3-1.2% for myocardial infarction, 6.7%-21.5% for moderate paravalvular regurgitation and 6-24.2% for post CABG or permanent pacemaker placement (Blackman DJ et al, DiMario C et al, Chieffo A et al).
In a clinical trial comparing BE and SE for device success the BE valve implantation resulted in a higher device excess than the SE, 95.9% versus 77.5%, respectively (Abdel-Wahab et al).
In the above study there was no difference in 30 day cardiovascular mortality rates: 4.3% in the SE valve group and 4.1% in the BE valve group.
Both valves have good flow characteristics.
SE valve use associated with high rate of residual paravalvular aortic regurgitation.
Surgical AVR has less paravalvular regurgitation rates then TAVR.
30 day mortality rates are higher and more variable in hospitals with low procedural volume than in hospitals with a high procedural volume.
Thromboembolic complications such as stroke, systemic embolism, valve thrombosis, and venous thromboembolism have been described after TAVR.
Presently, guidelines recommend the use of dual antiplatelet therapy early after TAVR.
Practice guidelines recommend clopidogrel in addition to aspirin for the first 3-6 months after TAVR in patients who do not have an indication for oral anticoagulation.
The procedure is complicated by life-threatening bleeding in 3-13% of patients and strokes occur in 1-12% at one year after TAVR.
In a study of rivaroxaban versus antiplatelet therapy there is a higher risk of death and thromboembolic complications and a higher risk of bleeding.
Among patients undergoing TAVR who did not have an indication for oral anticoagulation, the incidence of bleeding or thrombolic events at one year were significantly less frequent with aspirin than with aspirin plus clopidogrel administered for three months.
Among patients with severe aortic stenosis who were at low surgical risk, the rate of death, stroke, or rehospitalization at one year was significantly lower with TAVR than with surgery (Mack MJ).
