Reactive oxygen species (ROS) are chemically reactive chemical species containing oxygen.
Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen (O2) produces superoxide (•O−2), which is the precursor of most other reactive oxygen species:
Dismutation of superoxide produces hydrogen peroxide .
Hydrogen peroxide in turn may be partially reduced to hydroxyl radical (•OH) or fully reduced to water.
ROS are formed as the normal metabolism of oxygen and have important roles in cell signaling and homeostasis.
ROS are produced as a normal product of cellular metabolism.
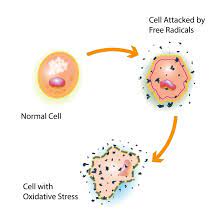
ROS levels can increase during times of environmental stress, resulting in significant damage to cell structures: oxidative stress.
Reactive oxygen species can damage the DNA and proteins in cells, and a majority of them arise in the mitochondria.
ROS are produced during biochemical reactions within the cell and within organelles such as mitochondria, peroxisomes, and endoplasmic reticulum.
Mitochondria convert energy for the cell into a usable form, adenosine triphosphate (ATP) by oxidative phosphorylation, involving the transport of protons (hydrogen ions) across the inner mitochondrial membrane by means of the electron transport chain.
Normally oxygen is reduced to produce water.
In about 0.1–2% of electrons passing through the chain oxygen is prematurely and incompletely reduced to give the superoxide radical (•O−2).
Another source of ROS production is the electron transfer reactions catalyzed by the mitochondrial P450 systems in ovary and testicle tissues.
To cope with this natural source of ROS, the steroidogenic tissues, ovary and testsis, have a large concentration of antioxidants such as vitamin C and β-carotene and anti-oxidant enzymes.
ROS damage to mitochondria, leads to cellular apoptosis or programmed cell death.
ROS are produced in immune cell signaling such as phagocytic cells such as neutrophils, eosinophils, and mononuclear phagocytes.
ROS formation can be stimulated pollutants, heavy metals, tobacco, smoke, drugs, xenobiotics, or radiation.
Ionizing radiation can generate damaging ROS intermediates through the interaction with water.
In the process of radiolysis, water loses an electron and becomes highly reactive: water is sequentially converted to hydroxyl radical (•OH), hydrogen peroxide (H2O2), superoxide radical (•O−2), and ultimately oxygen (O2).
The hydroxyl radical is very reactive and immediately removes electrons from any molecule in its path, turning that molecule into a free radical and thus propagating a chain reaction.
Hydrogen peroxide is more damaging to DNA than the hydroxyl radical, since its lower reactivity provides enough time for the molecule to travel into the nucleus of the cell, subsequently reacting with macromolecules such as DNA.
Superoxide dismutases (SOD) are a enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide.
Superoxide dismutases are an important antioxidant defense in nearly all cells exposed to oxygen.
Three forms of superoxide dismutase are present:
SOD1 is located primarily in the cytoplasm,
SOD2 in the mitochondria
SOD3 is extracellular.
SOD1 and SOD3 contain copper and zinc ions, while SOD2 has a manganese ion in its reactive center.
SOD genes are located on chromosomes 21, 6, and 4, respectively.
ROS plays a role in apoptosis, induction of host defense genes and mobilization of ion transport systems.
ROS help control of cellular function.
Platelets release ROS to recruit additional platelets to sites of injury.
ROS help recruit leukocytes.
Reactive oxygen species are implicated in inflammatory responses including cardiovascular disease, hearing impairment via cochlear damage induced by elevated sound levels, in ototoxicity of drugs such as cisplatin, and in congenital deafness,
mediation of apoptosis or programmed cell death and ischemic injury.
The brain metabolizes as much as a fifth of consumed oxygen, and reactive oxygen species produced by oxidative metabolism are a major source of DNA damage in the brain.
Harmful effects of reactive oxygen species on the cell :
damage of DNA or RNA
oxidations of polyunsaturated fatty acids in lipids- lipid peroxidation.
oxidations of amino acids in proteins
oxidative deactivation of specific enzymes by oxidation of co-factors
The brain metabolizes as much as a fifth of consumed oxygen, and reactive oxygen species produced by oxidative metabolism are a major source of DNA damage in the brain.
ROS is an induced antimicrobial defense, but in patients with chronic granulomatous disease who have deficiencies in generating ROS, are highly susceptible to infection by a number of microbes including Salmonella enterica, Staphylococcus aureus, Serratia marcescens, and Aspergillus spp.
Increased levels of ROS increase signaling through this mitochondria-associated antiviral receptor to activate interferon regulatory factor and nuclear factor kappa B (NF-κB), resulting in an antiviral state.
Respiratory epithelial cells induce mitrochondrial ROS in response to influenza infection, which leads to the induction of type III interferon and the induction of an antiviral state.
In host defense against mycobacteria, ROS play a role, in signaling controls, such as cytokine production, autophagy, and granuloma formation.
Reactive oxygen species are also implicated in activation, anergy and apoptosis of T cells.
ROS can damage lipid, DNA, RNA, and proteins, which may contribute to the process of aging.
With age, cellular components accumulate reactive oxygen species (ROS).
These free radicals can damage DNA, lipid, and protein and is able to drive cellular senescence, and is accompanied by a loss in efficiency of DNA damage repair mechanisms.
A major contributor to oxidative damage is hydrogen peroxide, which is converted from superoxide that leaks from the mitochondria.
ROS are produced as a product of normal cellular functioning, excessive amounts can cause deleterious effects.
Alzheimer’s disease is accompanied by an accumulation of oxidative damage.
Oxidative damage is a contributor to senescence, leading to cognitive dysfunction.
Accumulating oxidative damage can then affect the efficiency of mitochondria and increases the rate of ROS production.
The free radical theory of aging: oxidative damage initiated by reactive oxygen species is a major contributor to the functional decline that is characteristic of aging.
Exposure of spermatozoa to oxidative stress is a major cause of male infertility, manifesting sperm DNA fragmentation.
A high level of the oxidative DNA damage is associated with abnormal spermatozoa and male infertility.
ROS are constantly generated and eliminated in the biological system.
ROS are required to drive regulatory pathways.
With normal physiological conditions, cells control ROS levels by balancing their generation with their elimination by scavenging systems.
Excessive ROS can damage cellular proteins, lipids and DNA, leading to fatal lesions in the cell that contribute to carcinogenesis.
Cancer cells exhibit greater ROS stress than normal cells.
Chronic inflammation, a major mediator of cancer, is regulated by ROS.
High level of ROS can suppress tumor growth as a cell-cycle inhibitor and induces cell death as well as senescence by damaging macromolecules.
Most chemotherapeutic and radiotherapeutic agents kill cancer cells by augmenting ROS stress.
Modest levels of ROS are required for cancer cells to survive, whereas excessive levels kill them.
NADPH in tumors is greatly enhanced, functioning as a cofactor to provide reducing power in many enzymatic reactions for macromolecular biosynthesis.
Increased NADPH rescues cells from excessive ROS produced during rapid proliferation.
Cells counterbalance the detrimental effects of ROS by producing antioxidant molecules, which rely on the reducing power of NADPH to maintain their activities.
Most risk factors associated with cancer are related to the interaction of cells through the generation of ROS.
ROS activates transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), activator protein-1 (AP-1), hypoxia-inducible factor-1α and signal transducer and activator of transcription 3 (STAT3), leading to expression of proteins that control inflammation, cellular transformation, tumor cell survival, tumor cell proliferation, invasion, agiogenesis as well as metastasis.
ROS control the expression of tumor suppressor genes such as p53, retinoblastoma gene (Rb), and phosphatase and tensin homolog (PTEN).
ROS-related oxidation of DNA is one of the main causes of mutations, producing several types of DNA damage, including base modifications, single-strand breaks, protein-DNA adducts, and intra/interstrand DNA crosslinks.
Endogenous ROS produced via normal cell metabolism modify approximately 20,000 bases of DNA per day in a single cell.
ROS are a normal byproduct of aerobic metabolism and have an essential role in host defense and signaling.
Usually antioxidants prevent excess ROS from accumulating,
In the presence of increased oxygen tension or an exaggerated stimulus such as toxins or physiological stress, ROS production increases and outstrips anti-oxidant capacity.
Under the circumstances oxidative stress occurs, with inflammation, cell damage, and cell death.
ROS superoxide anions can activate nitric oxide and PaO2 exceeds 150 mmHg and can induce vasoconstriction in the coronary, retinal and cerebrovascular beds.
8-oxoguanine is the most abundant of oxidized nitrogeneous bases.
During DNA replication, DNA polymerase mispairs 8-oxoguanine with adenine.
It leads to a G→T transversion mutation, resulting genomic instability directly contributing to carcinogenesis.
Both exogenous and endogenous ROS have been shown to enhance proliferation of cancer cells.
Agents with potential to inhibit ROS generation can also inhibit cancer cell proliferation.
ROS can promote tumor cell proliferation, but a great increase in ROS is associated with reduced cancer cell proliferation by induction of G2/M cell cycle arrest; increased phosphorylation of ataxia telangiectasia mutated (ATM), checkpoint kinase 1 (Chk 1), Chk 2; and reduced cell division cycle 25 homolog c (CDC25).
A cancer cell can dies by: apoptosis, necrosis, and autophagy.
Excessive ROS can induce apoptosis through both the extrinsic and intrinsic pathways.
In the extrinsic pathway of apoptosis, ROS are generated by Fas ligand as an upstream event for Fas activation via phosphorylation.
In the intrinsic pathway, ROS function to facilitate cytochrome c release by activating pore-stabilizing proteins (Bcl-2 and Bcl-xL) as well as inhibiting pore-destabilizing proteins (Bcl-2-associated X protein, Bcl-2 homologous antagonist/killer).
The intrinsic pathway is also known as the caspase cascade and is induced through mitochondrial damage which triggers the release of cytochrome c.
DNA damage, oxidative stress, and loss of mitochondrial membrane potential lead to the release proteins stimulating apoptosis.
Mitochondrial damage is closely linked to apoptosis.
ROS is a driving force behind apoptosis, resulting in both apoptosis and necrosis, a form of uncontrolled cell death, in cancer cells.
ROS levels and apoptosis and autophagy are related.
ROS can also induce cell death through autophagy.
Autophagy is a self-catabolic process involving sequestration of cytoplasmic contents for degradation in lysosomes.
Autophagy helps regulate the cell’s health in times of oxidative stress.
Autophagy can be induced by ROS levels through many different pathways.
Autophagy activation in the cell in an attempt to dispose of harmful organelles and prevent damage, such as carcinogens, without inducing apoptosis.
Autophagy and apoptosis are two different cell death mechanisms brought on by high levels of ROS in the cells.
Reactive oxygen species (ROS) are regarded as unwanted by-products of oxidative phosphorylation in mitochondria.
It is proposed that substances which inactivate ROS, such as antioxidants, would lead to a reduction of oxidative stress and thereby produce an increase in lifespan.
ROS has a potentially lifespan-promoting role as redox signaling molecules transduce signals from the mitochondria to other parts of the cell.
Increased ROS within mitochondria may increase stress resistance and a long-term reduction of oxidative stress.
This reverse effect of the response to ROS stress has been named mitochondrial hormesis.
Mitochondrial hormesis is hypothesized to be responsible for lifespan extension and health-promoting capabilities of glucose restriction and physical exercise.
ROS and autophagy are connected and and a correlation is seen between excessive amounts of ROS leading to apoptosis.
The depolarization of the mitochondrial membrane is also characteristic of the initiation of autophagy.
Mitochondria damage begins to release ROS, and autophagy is initiated to dispose of the damaging organelle.
Extensive amounts of ROS and mitochondrial damage may also signal apoptosis.
The balance of autophagy within the cell and the crosstalk between autophagy and apoptosis mediated by ROS is crucial for a cell’s survival.
ROS can trigger activation of signaling pathways involved in cell migration and invasion: mitogen activated protein kinase (MAPK) family.
ROS have been shown to induce transcription factors and modulate signaling molecules involved in angiogenesis: MMP, VEGF and metastases.
There are close associations among ROS, chronic inflammation, and cancer.
ROS induces chronic inflammation by the induction of COX-2, inflammatory cytokines (TNFα, interleukin 1 (IL-1), IL-6), chemokines (IL-8, CXCR4) and pro-inflammatory transcription factors (NF-κB).
Such chemokines and chemokine receptors, in turn, promote invasion and metastasis of various tumor types.
Cancer cells with elevated ROS levels depend heavily on the antioxidant defense system.
Drug agents that abrogate the inherent antioxidant system result is an overall increase in endogenous ROS, which when above a cellular tolerability threshold, may induce cell death.
Normal cells appear to have a higher capacity to cope with additional ROS-generating insults than cancer cells do.
Elevation of ROS can be used to achieve the selective killing of cancer cells.
Radiation therapy relies on ROS toxicity to eradicate tumor cells.
Radiotherapy induces ROS-mediated cell death and mitotic failure.
Spikes of ROS are needed to correctly fold proteins in the endoplasmatic reticulum and low ROS levels may thus aspecifically hamper the formation of tumor suppressor proteins.
Physical exercise induces temporary spikes of ROS, suggesting its benefits for cancer patient prognosis.
ROS have a central role in epigenetic DNA demethylation.
