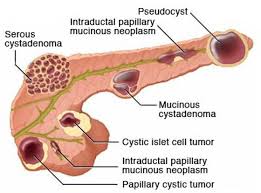
 Include benign serous cystadenomas, malignant cystadenomas, intraductal papillary mucinous tumors, inflammatory cysts and rarely cystic islet cell tumors and lymphoepithelial cysts.
Include benign serous cystadenomas, malignant cystadenomas, intraductal papillary mucinous tumors, inflammatory cysts and rarely cystic islet cell tumors and lymphoepithelial cysts.
Increasingly recognized, especially in asymptomatic and elderly patients due to the widespread use of high resolution imaging.
The incidence is estimated to be between 3 and 15%.
In autopsy studies pancreatic cyst were found in greater than 24%-50% of autopsy cases.
The incidence of pancreatic cyst is on the rise, and increases with age.
Pancreatic cysts are detected in 3% of abdominal CT scans and 20% of abdominal MRI scans.
Prevalence increases with age, with up to 10 – 40% of patients having pancreatic cysts over 80 years of age.
Pancreatic cysts are benign, with only subset that has malignant potential.
The overall risk of malignancy and pancreatic may be as low as 0.5 to 1.5% and the annual risk of progression is 0.5%.
Conversely, it is estimated that 15% of all pancreatic adenocarcinomas originate from mucinous cysts, and these cysts are the recognizable precursors of malignant transformation that can be identified on cross-sectional imaging.
Surgical resection is the only curative treatment of these cysts and carries a risk of major complications.
In a Korean study intraductal papillary mucinous neoplasm accounted for 41% of lesions, mucinous cystic neoplasms for 25.2%, solid pseudopapillary neoplasms for 18.3%, serious cystic neoplasms for 15.2% and others for 0.3 percent of pancreatic cysts (Yoon WJ).
In a Japanese study the expected mortality observed for pancreatic cancer was 22.5 times higher in patients with pancreatic cysts then the general Japanese population (Tada M).
In a American Gastroenterological Association review of asymptomatic neoplastic pancreatic cysts reported estimated incident risk of malignant tumor of the incidental pancreatic cyst at 0.24% per year with a prevalent more risk of 25% at the time of diagnosis (Sheiman JM).
Information indicates pancreatic cyst can be asymptomatic at the initial presentation, but they may develop into cancer, and malignant tumor risk is higher in patients with pancreatic cyst than in the healthy population without such pancreatic cysts.
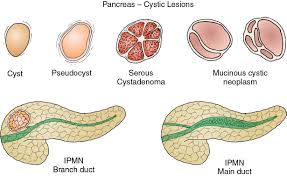
Pancreatic cysts are broadly classified as either non neoplastic or pancreatic cystic neoplasms.
There are more than 20 types of epithelial and non-epithelial pancreatic cysts.
The two most prevalent benign pancreatic cysts are pseudocysts and serous cyst adenomas which account for 15 to 25% of all pancreatic cysts.
The two types of mucinous cysts: intraductal papillary mucinous neoplasm, and mucus cystic neoplasm are the predominant premalignant cystic lesions and account for approximately 50% of cysts that are found incidentally on imaging.
Solid pseudocapillary democrats harder neoplasm and cystic pancreatic neuroendocrine tumors are less common malignant cystic neoplasm.
Classified as true cysts or pseudocysts based on whether an epithelial lining is present or not.
Non neoplastic pancreatic cysts are better characterized as non epithelial type with pancreatic pseudocysts the most common, and epithelial cysts with retention cysts being the most common.
Non neoplastic cysts of the pancreas are benign without malignant potential, but they may be indistinguishable from pancreatic cystic neoplasms.
Non neoplastic cysts of the pancreas are rare, they should be recognized to avoid unnecessary pancreatic resection.
Non epithelial cysts include pancreatic pseudocysys and infectious related cysts, whereas epithelial cysts include retention cysts.
Pancreatic cystic neoplasms are mainly divided into mucinous and nonmucinous cystic lesions.
Pseudocysts account for 75% of pancreas cysts with true cysts making up 25% of such lesions.
Pseudocysts follow acute or chronic pancreatitis, and typically appear as single or multiple unilocular cyst that may contain debris.
In the absence of an antecedent pancreatitis the diagnosis of the pseudocysts should be made with great caution.
Most pseudocysts resolve spontaneously, and intervention is warranted only in patients that are symptomatic.
Serous cysts are benign, slow growing and affect women primarily in the 5th to 7th decades of life.
A central scar on CT or MRI is pathopneumonic, but observed only in 30% of cases.
Most cases are asymptomatic, although large cystadenoma can cause pain, pancreatitis, and biliary obstruction.
Mucinous cystic neoplasms are less common than mucinous cysts, and are characterized by ovarian like-stroma, almost exclusively affects women in the 4th to 6 decades of life, are single thick walled, mostly unicellular in the distal pancreas.
Mucinous cystic neoplasms have no communication with pancreatic ducts, and also rare, the presence of peripheral eggshell calcifications is diagnostic.
The risk for high-grade dysplasia or cancer in patients with mucinous cystic neoplasm is as high as 30 to 40%, but when pathgnomonic ovarian type stroma is confirmed, only 5 to 15% of lesions contain invasive cancer.
Intraductal papillary mucinous neoplasms (IPMNs) are the most common type of mucus cystic lesions, with equal sex distribution, a peak incidence between fifth and 76 decades of life.
IPMNs arise from ductal cells, often multifocal, and located throughout the pancreas.
IPMNs are classified according to duct involvement: main, branch, or mixed duct.
A bulging mucin extruding fish mouth papilla is a pathognomic finding for main duct IPMDs seen on endoscopy.
The risk of malignant transformation depends on the histologic/anatomical sub types: for branch duct IPMNs 1 – 38%, and 33 to 85% for main duct or mixed type IPMNs.
Solid pseudopapillary neoplasm most often develop in women in the second or third decade of life,with lesions, located throughout the pancreas, are well demarcated with both solid and cystic components, and have a low but variable metastatic potential.
Cystic pancreatic endocrine neoplasm arise from pancreatic endocrine cells that undergo cystic degeneration from a pancreatic neuroendocrine tumor.
10% of these lesions, arise in multiple endocrine neoplasia type1.
More than 80% of cystic pancreatic endocrine, neoplasms express somatostatin receptors, which can be detected by PET scans with octreotide tracers.
Establishing a cyst type is essential to manage and assist risk assessment of pancreatic cysts.
Accurate classification by imaging and demographic data results occur in 70 to 80% of cysts.
When the diagnosis of a cyst type is equivocal endoscopic ultrasound with fluid or fine needle aspiration is helpful.
Small cysts without distinctive features are generally presumed to be mucinous and managed accordingly.
In evaluating pancreatic cysts, the purpose is to classify them as either benign lesions without malignant potential or lesions associated with low, intermediate, or higher risk of advanced neoplasia.
Cysts that are unequivocally benign on imaging such as serous cyst adenoma, and pseudocysts require no further investigation as these are managed primarily on the basis of symptoms.
Low risk cysts with no risk or minimal risk of current advanced neoplasia in low risk of a future malignant regression are mostly small mucinous cysts, predominantly branching IPMNs.
Intermediate risk cysts have a minimal risk of current advanced neoplasia, but a moderate risk of future malignant progression.
High risk cysts are associated with a high probability of current advanced neoplasia.
Most intermediate and high risk lesions are mucinous cysts, with fewer cases of solid pseudopappilary tumors and solid pancreatic endocrine tumors, and rare cases of cystic degeneration, of carcinoma.
The initial evaluation of cysts starts with imaging studies, followed by symptom history and laboratory tests.
Concerning high risk stigmata imaging studies for malignancy include biliary obstruction, dilation of the main pancreatic duct of greater than 10 mm, and a solid enhancing nodule of 5 mm of greater.
Features worrisome for neoplasia include a cyst greater than 3 cm in diameter, main duct dilation of 5 to 10 mm, a contrast, enhancing mural nodule of less than 5 mm, an enhanced thicken cyst wall or septations, lymphadenopathy, change in the caliber of the pancreatic main duct with distal pancreatic atrophy, and an increase in size greater than 20% or approximately 2.5 mm in diameter per year.
The absence of these imaging findings noted above is consistent with a low risk of malignant potential.
A minority of cysts are symptomatic.
The presence of jaundice caused by a biliary obstruction is a high risk feature risk, clinical feature, and pancreatitis due to obstruction of a pancreatic duct is an intermediate risk factor related to the cyst.
A serum CA 19–9 marker is associated with increased risk as is a newly increased Hgb A1C.
Endoscopic ultrasound can enhance stratification education with intermediate risks and can affirm the diagnosis of benign a low risk cysts.
Patients with high risk cysts endoscopic ultrasound can help establish a preoperative diagnosis of suspected advanced neoplasia.
Endoscopic imaging is effective for identifying ductal communication, has a high sensitivity to detect small mural nodules, and can help identify the pathognomic fish mouth papilla.
Endoscopic imaging with contrast is valuable to detect the presence of epithelial nodules, which is probably the strongest predictive risk factor for malignant transformation, aside from main duct dilatation.
Endoscopic imaging allows for fine needle aspiration of solid components and for biopsy of intracystic lesions.
Fine-needle aspiration of cysts allows, obtaining material for the cytological diagnosis, but it’s yield is low.
Measurement of cyst amylase, CEA and glucose does not differentiate between neoplasia, but can aid in diagnosis.
An elevated cyst amylase level suggests communication with the pancreatic ductal system and ischaracteristic of pseudocysts and IPMNs:a Low MLS level in cyst fluid essentially rules out a pseudocyst.
Pancreatic cyst CEA levels are elevated in 75% of mucinous cysts and very low levels almost rules them out.
A low level of glucose in pancreatic cystic fluid of 50 to 80 mg/mL is 90 to 94% accurate in distinguishing mucinous from non-mucinous cysts.
DNA in cystic fluid can detect mutations associated with specific neoplasms.
The presence of VHL mutation is nearly 100% specific for serous cystadenoma but it’s seen only in 25 to 50% of cases.
The KRAS mutation is more than 95% specific for either type of mucinous cyst with the sensitivity of 60 to 70%.
Mutations in GNAS are specific for IPMNs and detected in 30 to 60% of cases.
Cystic pancreatic fluid absence of a VHL mutation combined with the presence of a KRAS or GNAS mutation is nearly 100% specific for mucinous cysts, with an accuracy of 97%.
Detection of a CTNNB1 mutation is highly specific for solid pseudopapillary tumors, and the presence of MEN1 mutation has a high specificity for a cystic pancreatic endocrine neoplasm.
Cyst fluid mutational status with abnormalities in oncogenes and tumor suppressant gene such as TP53, CDKN2A, SMAD4 and CTNNB1 and genes involved in the mTOR pathway PIK3CA, PTEN, and AKT1 are most commonly found in mucinous cysts with high-grade dysplasia or cancer.
The definitive or presumptive diagnosis of a mucinous cyst may include management by surgical intervention, watchful waiting, surveillance or refraining from further action.
A management plan includes estimating the risk of malignant transformation, and the patient’s overall health outlook.
Patients with high risk cysts, and an acceptable operative risk should undergo surgical resection.
Minimally, invasive, surgical approaches, are being increasingly used.
For intermediate risk cysts, the majority are presumed to be mucinous and endoscopic ultrasound and cyst fluid analysis can help in management of these cases: lesions with worrisome cytologic features indicating neoplasia, or high risk genomic alterations in fluid favors surgical resection, whereas their absence, justifies surveillance.
IPMNs are often multifocal and require segmental resection of the affected gland.
For low risk cyst management, surveillance is recommended with follow up every six months in the first year and then yearly.
Surveillance is performed with MRI or magnetic residence cholangiopancreatography or with contrast enhanced CT.
Cyst stability indicates less than a 20% increase in the greatest diameter or growth of less than 2.5 mm per year.
Faster growth or the development of new intermediate risk or high risk features warrant, considering reconsideration for endoscopic ultrasonography with without fine needle aspiration or biopsy, or surgical resection.
