 Refers to the constellation of microbes living in and on the body.
Refers to the constellation of microbes living in and on the body.
Refers to the collective genomes of microbes, composed of bacteria, bacteriophage, fungi, protozoa and viruses that live inside and on the human body.
Humans are colonized by trillions of viral, fungal, bacterial, and eukaryotic microbes, collectively referred to as the microbiome, which are present on all barrier surfaces.
There is enormous intrapopulation variation of the human microbiome among individuals.
Refers to a community of all micro organisms inhabiting a specific site in the the body, including pathogens.
Microbiome denotes not only micro organisms, but also their collective genomes.
Microbial genes are involved in the metabolism of carbohydrates, amino acids, and the production of vitamins.
The microbiome contributes to the balance of circulating nutrient and metabolite levels via production or transformation of neurotransmitters, short chain fatty acids, amino acids, and secondary bile acid that can either be absorbed or reabsorbed by the host to enter the circulation.
The metabolism of the microbiome is referred to as the metagenome.
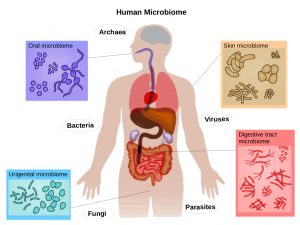
Microbial communities in and on the human body, include the skin, nasal passages, gastrointestinal tract, and urinary tract.
Refers to the total number of microorganisms and their genetic material.
Represents the complex collection of micro organisms within and on individuals, their genomes, and collective functions.
The disappearance of constituents of the microbiota owing to improved hygiene, antibiotic pressure, and smaller family sizes is hypothesized to contribute to the contemporary increases in autoimmune and allergic diseases.
The bacteria and microorganism are characterized as the microbiota, that inhabits the epithelial barrier surfaces of the human body and influences a wide range of physiological functions, including metabolism and immunity.
The microbiota as important role in the training and development of the immune system.
Certain commensal bacterial species are able to induce distinct immune cell populations to polarize and play either pro-inflammatory or anti-inflammatory roles: some lead to immune suppression.
Has an extensive role in physiology and composition is influenced by an individual’s genetics, lifestyle , incidence of disease and use of antibiotics.
Because antibiotics markedly affect microbial composition, an even transient perturbation during critical developmental periods may compromise both immune tolerance and inflammatory responses.
The average human has 100 trillion microbes in the gastrointestinal tract.
There are more than 50 microbial species in the vaginal tract., while in comparison, the gut is populated with more than 800 species of microbes, the majority of which are excreted in feces, and a number of which are well equipped to be pathogenic.
99% of the microbial mass in the body is found within the gastrointestinal tract.
There are 10 times more microbes in the human gut, then there are cells in the human body.
Crucial to health and well-being.
Higher gut microbial diversity is associated with improved survival in patients undergoing hematopoetic poetic stem cell transplantation.
The nascent gut microbiota of infants is essential in establishing proper immune function and that disruptions to this community results in early dysfunction and subsequent development of asthma.
The microbiology is resilient and has plasticity, making it more mutable than human cells.
The microbiome resiliency is demonstrated in health by being resistant to temporary insults such as travel, diet, and antibiotics, maintaining a relatively stable steady state.
The micrbiome is malleable and can be modified by dietary and other directed therapies.
There is interindividual variability in composition and metabolic capacity playing an important role in interactions with the environment, with the development of disease as well as response to treatment and development of adverse events.
Partly determined by the host genome.
Benefits of human microbiome include: differentiation of host mucosa, food digestion, regulation of metabolism, detoxification of environmental chemicals, development and regulation of immune system, prevention of invasion and proliferation of pathogens.
When disturbed, the human microbiome associated with disease, such as periodontitis, inflammatory bowel disease, and antibiotic associated diarrhea.
Enterobacteriaceae was found in significantly high levels in patients with diverticular disease.24
Depletion of Clostridium cluster IV, Clostridium cluster IX, Fusobacterium, and Lactobacillaceae are found in patients with symptomatic diverticula disease.
Body site is one of the major determinants of variation in composition diversity.
After only 10 days of treatment with systemic antibiotics, the gut microbiota can be altered for up to 1 year.
There are three types of microbial communities on the skin: each characteristic of either dry, moist, or sebaceous environments.
Propionibacterium acnes, commensal staphylococci, Corynebacterium species, and Propionibacterium phage explain the greatest variation between these community types, while fungi and other eukaryotic microbes a relatively rare.
There are gut microbiomic differences between obese and non-obese individuals, and between individuals with inflammatory bowel diseae and healthy people.
Alterations in the microbial community is associated with diseases that range from infectious to inflamatory and metabolic diseases suggesting pathogenesis of multifactorial conditions.
Associated with Clostridium difficile infection, inflammatory bowel disease, rheumatoid arthritis, diabetes, and obesity.
Use of broad-spectrum antibiotics can disrupt gut microbiota and is an independent risk factor for transplant related mortality after allogeneic stem cell transplant.
The oral microbiome has approximately 700 bacterial species that have been identified in the human oral cavity.
Bacteria may colonize various sites in the human oral cavity, such as the gingiva, dental plaque, and tongue.
Inappropriate antibiotic exposure may be linked to an increased risk of colon cancer by alterations in the microbiome.
Studies have shown an association between antibiotic use and an increased risk for colon cancer.
A Swedish population study from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons, found that moderate use of antibitotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
Reports from the vaginal, lung, and oral cavity are closely related with relevant tumor occurrence and progression: microbiota tumor interactions have qualified microbiota as a promising biomarker a therapeutic target for diverse tumors.
There is evidence of involvement of the intratumoral microbiota on oncogenic behaviors in pancreatic, lung, and breast cancers.
Intra-tumoral bacterial load is associated with poor survival and patients with nasopharyngeal carcinoma and is negatively associated with T-lymphocyte infiltration.
Pathogenic bacteria, including gram-negative species are not seen in the normal host, but may emerge in the elderly, as well as in patients in nursing homes or hospitals and those with nasogastric tubes.
There is a relationship of the oral microbiome and esophageal cancer.
There is a relationship of the vaginal and uterine microbiome and endometrial cancer.
A relationship exists between the gut microbiome and the efficacy of checkpoint inhibitors.
There is growing evidence that mood disorders may be related to overall inflammation and to changes in the microbiome
The oral pathogens Porphyromonas gingivalis and Aggregatibacter actinomyccetemcomitans are associated with an increased risk of pancreastic cancer, while an abundance of Fusobacteria and its genus Leptotrichia are associated with decreased risk.
Carrying Neisseria in abundance in the oral cavity associated with a lower incidence of head and neck canccer.
Neisseria found in lower concentrations in the mouth of smokers, suggesting a link to cancers.
Fusobacterium nucleatum, normally found in the mouth and is associated with periodontal disease and is often present in colorectal cancer, and is related to an increased risk.
Fusobacterium found in colorectal cancers and liver metastases, suggesting the microbiome is a consistent feature of colorectal cancers.
