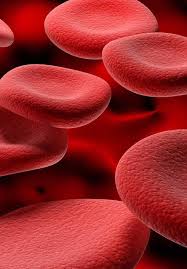
 Biconcave shape provides optimal area for respiratory exchange.
Biconcave shape provides optimal area for respiratory exchange.
There are more than 200 billion erythrocytes requiring more than 20 mg of elemental iron.
Diameter 7.2 microm, with usual variation of 6.8=7.5 microm, with an extreme size limits 6.2=8.2 microm.
Normal size RBCs referred to as normocytic, larger than normal cells are macrocytic and smaller than normal cells are microcytic.
Macrocytic RBCs are greater than 8.2 microm, while microcytic cells are smaller than 6.2 microm in size.
Anisocytosis refers to increased variation in cell size, and is common in severe anemias.
Poikilocytosis refers to the presence of multiple shapes that red blood cells can assume other than the normal round biconcave appearance.
Poikilocytes can appear as many sheets including oval, pencil like, and teardrop.
Red blood cells at first in the bone marrow have nucleus but those red blood cells that are active in blood lose their nucleus and thus they have no nucleus and no chromosomes.
Specific poikilocytes have nomenclature such as acanthocytes, blister cells, burr cells, crenated erythrocytes, echinocytes, elliptocytes, keratocytes, ovalocytes, pyknocytes, schistocytes, sickle cells, spiculated erythrocytes, spherocytes, stomatocytes, target cells, and teardrops.
Spherocytes are seen in immune mediated hemolysis and hereditary spherocytosis.
Spherocytes are irregularly contracted erythrocytes, and bite cells can be identified in cases of oxidated hemolysis related to severe burns or G6PD deficiency.
Its respiratory protein, hemoglobin, performs oxygen-carbon dioxide transport as its function.
A simple erythrocyte, enucleated and without mitochondria, contains more than 750 proteins.
Red blood cells are containers for hemoglobin and are critical for oxygen transport and delivery from lungs to tissues throughout the body supporting aerobic metabolism.
Red blood cells do not contain mitochondria and are therefore entirely dependent on anaerobic glycolysis for their energy requirements.
Red blood cells have limited ability to metabolize fatty acids and amino acids and lack mitochondria for oxidative metabolism.
Energy for metabolic process is generated primarily through the breakdown of glucose.
The pathway of erythrocytic glycolysis is that subdivided into it the major anaerobic Embden Meyerhof glycolytic pathway and three other supplementary pathways.
The Embden-Meyerhof pathway uses glucose and generates adenosine triphosphate (ATP).
The Embden-Meyerhof pathway is also essential in maintaining pyridine nucleotides in a reduced state to support the methemoglobin reductase pathway and 2,3 -DPG synthesis.
The RBC membrane and metabolic mechanisms facilitate oxygen transfer and delivery function of hemoglobin.
Its membrane, hemoglobin and proteins involved in metabolic pathways interact to modulate oxygen transport, protect hemoglobin from oxidation and maintain osmotic environment of the cell.
Requires energy during its intravascular lifespan to maintain glycolysis, electrolyte gradient between plasma and red cell cytoplasm via ATP membrane pumps, to synthesize glutathione and other metabolites, for purine and pyrimidine metabolism, to maintain hemoglobin’s iron in the reduced f2242ous state, to protect metabolic enzymes and membrane proteins and hemoglobin from oxidative denaturation and preserve red blood cell membrane phospholipid asymmetry.
Because the mature red blood cell has no nuclei and mitochondria it can not generate energy via the Krebs cycle.
Depends on anaerobic conversion of glucose by the Embden-Meyerhoff pathway for generation and storage of high energy phosphates.
Possesses a glycolytic bypass for production of 2,3 bisphosphoglycerate (2,3 DPG), the Rapoport-Luebering shunt, which bypasses the phosphoglycerate kinase step and synthesizes and regulates 2,3-DPG levels that decrease hemoglobin’s affinity for oxygen.
In RBCs the extrinsic protein spectrin, in conjunction with the protein actin form a contractile network under the cell membrane that provides cellular resistance so that the red blood cells do not become distorted during movement through the circulation.
RBC fragility test is based on the principle of osmosis demonstrating changes in th erythrocyte membrane.
In arterial flow erythrocytes are lifted away from the endothelium via a hemodynamic process called axial migration.
After release from the bone marrow red blood cells reduce their volume, and total hemoglobin content.
With aging the cytoplasmic enzymes are catabolized with increased membrane rigidity and subsequently phagocytosis and ultimately destruction of the red cell occurs.
Hemoglobin comprises approximately 95% of the RBC total cellular protein.
Oxygen transport to the tissues and carbon dioxide transport from tissues are accomplished by the heme component of hemoglobin.
Research models suggest that there is a threshold for the mean cellular hemoglobin concentration below which most red blood cells are cleared from the circulation.
In anemia of chronic disease and in thalassemias red blood cells with smaller volumes and lower hemoglobin concentrations persist in the blood stream and may suggest that the delay in clearance is a compensatory process for less efficient erythrocyte production in these disorders.
