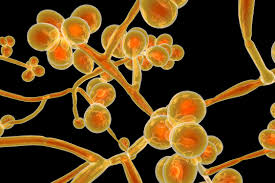
 Candida auris (CA) is a species of fungus that grows as yeast.
Candida auris (CA) is a species of fungus that grows as yeast.
CA is a budding yeast that unlike C. Albicans rarely forms pseudo hyphae or hyphae.
It is subdivided into five clades I-IV, with distinct geographic distributions.
It thrives in high salt and high temperature conditions, and its adaptability may account for its persistence in harsh environments, and perhaps related to environmental adaptation to climate change.
It may be among the first pathogenic fungi to have emerged in humans because of climate change.
The organism has the ability to form multi layer bio films when exposed to human sweat and resides within hair follicles in penetrates deep skin layers, evading clinical detection.
This adhesion in biofilm formation, colonization of skin and medical devices and virulence during invasive infections explains its seriousness.
It is one of the few species of the genus Candida which cause candidiasis in humans.
It has the ability to adhere to human skin and inanimate objects, and to persist, causing difficult to control outbreaks in healthcare facilities.
It has a high morbidity and mortality associated with invasive infections and resistance to several classes of antifungal agents have been demonstrated.
Often, candidiasis is acquired in hospitals by patients with weakened immune systems.
C. auris can cause invasive candidiasis, fungemia, in which the bloodstream, the central nervous system, and internal organs are infected.
It can spread hematogenously to distal anatomic sites and has been isolated from CSF, plural, pericardial, biliary and peritoneal fluids, and can cause myocarditis, pericarditis, meningitis, hepatosplenic infection, osteomyelitis, UTIs, endopthalomitis, ear infections, and wound infections along with donor derived disease after lung transplant ion.
Case fatality of C. auris associated candida invasive candidiasis is between 30 and 60%.
It is easily misidentified as other Candida species.
It can remain viable for at least two weeks on plastic surfaces and for months on the skin.
It is more likely to resist antifungal agents, then other candida species.
Its spread within healthcare facilities and from one facility to another.
Most outbreaks of C. auris cases occur in high acuity post-acute care facilities.
It can easily travel from patient to patient or from a contaminated surface, such as a door knob to a patient.
As many is 90 to 95% of patients colonized with C auris are asymptomatic, but can spread the yeast to others.
The most common sites of colonization are: the skin, the axilla, and groin:colonization of the G.I. or Euro general tract occurs less often.
Its colonization pattern differs from other candida species which predominantly colonize the G.I. tract.
Involvement of the mucosal areas, oral thrush, esophageal, candidiasis, and vulvovaginal candidiasis are infrequent with auris.
It has been identified in the environment and an animals.
Risk factors for colonization and infection are similar to those for candida species: advanced stage, diabetes, recent surgery, indwelling medical devices, immunosuppression, food, end stage kidney disease on dialysis, and received a broad-spectrum antimicrobials, including antifungals, parenteral nutrition, mechanical, ventilation, hemodialysis, neutropenia, glucocorticoid juice, receipt of an organ transplant, coronavirus disease, and previous skin colonization by C. auris.
Hospitalizations within the prior year is a risk factor.
C. auris clinical presentation ranges from asymptomatic colonization to severe infections.
Colonization occurs on the skin, urinary, gastrointestinal and respiratory tracts prior to the development of infection.
The most common severe presentation of the infection is a fungemia, that may be related to the presence of an intravascular catheter.
The mortality rate for C auris candidiasis systemic aainfection involving the blood, brain, and heart or other parts of the body is thought to be as high as 60%.
C auris can survive in water and saltier environments, then other fungal pathogens.
Studies suggest 5 to 10% of patients colonized with C auris go on to develop an invasive infection.
It has multiple drug resistance.
More than 90% of C. auris isolates are resistant to fluconazole, and a range of 3–73% of C. auris isolates are resistant to voriconazole, while other triazoles of posaconazole, itraconazole, and isavuconazole have better activity.
Of C. auris isolates, 13% to 35% were reported resistant to amphotericin B.
30–60% of people with C. auris fungemia die.
There is currently no vaccine for Candida auris.
The C. auris genome encodes several genes for the ABC transporter family, a major facilitator superfamily associated with multiple drug resistance.
Its genome also encodes virulence-related gene families such as lipases, oligopeptide transporters, mannosyl transferases, biofilm formation, and transcription factors which facilitate colonization, invasion, and iron acquisition.
C. auris has distinct genotypes exist in different geographical regions with substantial genomic diversity.
Global warming will lead to selection of fungal lineages that are more thermally tolerant.
Diagnosis is difficult because it is phenotypically similar to other yeast species and frequently misidentified by testing.
Detection requires mass spectroscopy, or molecular identification, based on genetic sequencing.
Culture based testing of specimens is the cornerstone of lab diagnosis.
Isolation of C. auris in culture is important for antifungal susceptibility pattern and reporting, and it can be readily cultured from blood, urine, sputum, and other body fluids.
morphologically it cannot be distinguished from other candidate species.
Screening with axillary and groin swabs for culture or polymerase chain reaction testing is proposed for preventing outbreaks.
Treatment is complicated by resistance to anti-fungal agents.
Resistance to antifungal agents is Clade specific.
Resistance to fluconazole appears to be acquired and approximately 90% of strains from all clades except for Claude II are resistant.
For infections in adults and children two months in age or older is recommended to use echinocandins as empirical or initial therapy pending susceptibility testing.
Resistance to echinocandins is uncommon.
Clinical resistance amphoteric B is reported in about 30% of isolates.
Production of interleukin 17A and interleukin 17 F by innate and adaptive lymphoid cells decrease skin colonization and invasion by C. auris consistent with production of interleukin 17 signaling and combating candida at mucocutaneous barriers.
Agents that target interleukin 17 pathway that are often used to treat psoriasis, may heighten the risk of C. aureus skin colonization.
C. auris invades phagocytosis and killing by neutrophils, which differ significantly from that of C. albicans.
Flucanazole resistance rates of 86 to 100% have been reported.
43% of isolates are resistant to amphotericin B.
The CDC recommends the use of echinocandins as empirical or initial therapy for C. auris pending results of accessibility testing.
Echinocandins resistance is the least prevalent occurring in 2 to 5% of isolates,and or the first line antimicrobials for invasive C auris infections.
Agents include caspofungin, micafungin, anidulafungia, and amphotericin B.
Mortality associated with C auris fungemia ranges from 30 to 72%.
C. Auris is often resistant to azoles and oral treatment options are limited.
Transmission of C. auris can occur in healthcare settings within 3 to 4 hours of contamination of the environment or equipment.
This requires contact precautions, enhanced barriers, universal use of gown, and gloves during contact with patients or their environment and placing of individuals in isolation or grouping patients with C. auris colonization or infection.
