 The ACS estimated they were 81,180 new cases and 17,100 deaths from bladder cancer in the US in 2022.
The ACS estimated they were 81,180 new cases and 17,100 deaths from bladder cancer in the US in 2022.
Bladder cancer is the 10th most common cancer globally and the fourth most common in men.
More than 430,000 patients diagnosed worldwide/year.
The number of bladder deaths have remained relatively unchanged since the 1970s.
Incidence rates for bladder cancer have not changed for 40 years.
Fourth leading cause of cancer related deaths among men aged 80 years or greater.
The fourth most common neoplasm in males and twelfth most common malignancy in females.
Fourth most common cancer and eigth leading cause of cancer death in men.
Fifth or 6th most prevalent cancer in the US.
Represents about 2.8% of all cancer deaths.
4.7% of all new cancers.
Estimated 600,000 people living with bladder CA in the US.
Males:females 3:1 with median age 65 years at diagnosis.
40% over the age of 70 years.
Median age at diagnosis 73 years.
Rare under age 40 years.
Incidence is increasing.
Incidence rates in white individuals aged 50 years or more from 123.8-142.2 per 100,000 person years in men and from 32.5-33.2 per 100.000 person years in women, with similar patterns in other ethnic and racial groups.
Incidence is higher in industrialized countries rather than in developing nations.
Incidence increases with age.
Lifetime risk is 2.4%.
Diagnosis increases with age, peaking at 75-84 years.
Incidence and mortality higher in men than women -about 3 fold.
Represents 4.6% of new cancer diagnoses.
Increased incidence in whites.
White individuals are diagnosed about twice as often as African Americans and Hispanic Americans.
Chronic bladder infections and family or personal history of bladder cancer predisposes to the development of bladder cancer.
Has a high mutational frequency, up to 93% of advanced cases.
Molecular pathogenesis involves activated mutations in the RAS-MAPK and PI3K-AKT pathways, frequently in the FGFR3, H-RAS, and PI3KCA genes.
Increased risk in individuals with gene mutations encoding for GNT, NAT, RB1, PTEN and also those with the Lynch syndrome.
most patients with advanced bladder cancer, have genetic alterations with cycling dependent cycling dependent kinase inhibitor CDKNA, FGFR3 and PIK3CA and ERBB2.
First-degree relatives of patients with bladder cancer had at least a 50% greater risk of developing disease than the general population.
Patients typically present with gross hematuria or microscopic hematuria, a frequent urge to urinate, painful urination, and or lower back pain.
Patients are frequently mismanaged.
At the time of diagnosis, most patients present with non-muscle invasive bladder cancer, which has a 50 to 70% rate of superficial recurrence and the 10% to 30% rate of progression to muscle – invasive bladder cancer.
Approximately 75% of bladder cancer patient present with non – muscle-invasive bladder cancer with 70% having Ta disease, 20% having T1, and 10% have Tis.
Seventy-five percent of patients initially present with noninvasive bladder cancer—in situ disease (Tis) or T1 tumors that have invaded only the lamina propria, a subepithelial connective tissue,
Approximately 25% of patients present at first diagnosis with either muscle-invasive bladder cancer or metastatic disease.
1-6% of low-intermediate risk patients develop progressive disease, while 17-45%of high risk non-muscle disease treated with BCG develop progressive disease.
Once tumor invades the muscularis propria, it is muscle-invasive disease, and progression to incurable metastatic disease is expected, unless the patient receives definitive local therapy.
Muscle -invasive bladder outcome is poor with less than 10% survival at 5 years.
5-year survival rates decrease for advanced disease: In situ 95.7%, localized 70.1%, regional 35.2%, distant/metastatic 5% (NCI).
90% cases present with hematuria.
Patients with advanced disease may have pelvic bone pain, lower extremity edema and or flank pain.
On rare occasions a palpable mass present.
5yr survival 77.3%.
Survival is lower in blacks than Caucasians, Hispanics, and Asian Pacific islanders.
Survival decreases with advancing stage, from 95.9% of patients diagnosed with in-situ disease to 70.2% with localized disease, 34.5% with regional disease, and only 5.2% with metastatic disease surviving at least 5 years.
Localized disease accounts for 34% of new cases, regional and distant disease accounts for 7% and 4%, of new cases, respectively.
Because of frequent recurrences, requirement of intensive surveillance and frequent surgeries and long natural history of superficial tumors makes this lesion the most costly malignancy in the Medicare system from diagnosis to death.
A prostate stem cell antigen(PSCA) genetic variation raises risk of bladder cancer by 30-40%.
The PSCA gene missense variation raises the risk of bladder cancer.
Mortality per case is double that of prostate cancer and similar to breast cancer.
Annual incidence less than one-third of prostate cancer.
80% of newly diagnosed patients are 60 years or older.
Black patients are diagnosed at younger age than other patients.
Risk of diagnosis at an advanced stage increases by 1% per year of age and risk of death increased by 4%, 40% and 84% for older age, black and females, respectively.
Approximately 20% of bladder cancer patients die each year.
The median survival for patients with metastatic is 1.5years, and this number has not significantly changed during the last two decades.
Mortality of all stages has stagnated at approximately 50%.
Most cases of cancer related death is the result of systemic disease rather than from the primary tumor.
Prognosis based on pathologic stage, tumor grade, tumor size, presence of associated carcinoma in situ, and multicentricity of lesions.
Approximately 51% of newly diagnosed cases are in situ.
Approximately 35% of all bladder cancer cases are localized and have an overall five-year relative survival of 70%.
Regional and distant disease account for 7% and 4% of new cases, respectively.
5-year disease specific survival after cystectomy 50-60%.
Gold standard for detection and suspicious lesions is biopsy by cystoscopy.
All patients initially undergo transurethral resection of a bladder tumor for diagnostic and staging purposes.
Muscle invasive bladder cancer arises by the progression from urothelial dysplasia to flat carcinoma in situ and high-grade noninvasive lesions, where accumulation of genetic alterations cause invasive disease.
Alterations of genetic tumor suppressor genes responsible for cell cycle control include: p53, p16, and Rb.
Biopsy allows patients with non muscle invasive bladder cancer to be stratified into low, intermediate, and high risk groups based on the probability of recurrence and progression.
Transitional cell carcinoma cancer accounts for 90% of all bladder cancers and arises from transitional epithelium.
Other types of bladder cancer include squamous cell carcinoma accounting for 1-2% of cases, adenocarcinoma 1%, sarcoma less than 1% and small cell carcinoma of 1%.
Squamous differentiation, perineural invasion and the presence of lymphovascular invasion impair prognosis.
Bladder neck tumors, and tumors within the prosthetic urethra, and those at the ureteral orifices are difficult to completely reset and to accurately stage.
Bladder cancer has the third highest rate of mutations, behind lung cancer and melanoma.
These tumor mutations may generate neoantigens that are recognized by activated tumor T cells.
Cystectomy should be offered to patients with adverse features such as flat lesions, diffusely infiltrating tumors, with involvement of the bladder neck, ureteral openings, and prostatic urethra.
85% present with hematuria.
Approximately 90% are transitional carcinomas.
One third of patients have smoking history.
Tobacco smoking is the best established risk factor for both men and women.
Tobacco smoking is the most important risk factor in developing bladder cancer.
Approximately 50% of cases attributed to smoking.
Tobacco carcinogens are filtered by the kidneys resulting in bladder cell damage due to concentration of such agents.
Cancer of bladder 3 times more common in smokers than nonsmokers.
Certain genetic syndromes, most notably the Lynch syndrome, may also predisposed to urothelial cancer.
Causative factors: occupational exposure to chemicals such as aniline dyes, benzidine, xenylamine, cigarette smoking, analgesic abuse, bacterial and parasitic infections, pelvic irradiation, and chemotherapeutic agents such as cyclophosphamide.
Increased risk in painters, hairdressers, machinists, printers, truck drivers, and dye workers.
Risks increased in smokers with industrial exposure.
Increased risk with exposure to cyclophosphamide chemotherapy, pelvic radiation and long term pioglitazone.
Cystoscopy the gold standard for diagnosing bladder tumors.
70% present as a superficial tumor-Ta, T1 or Tis.
50-70% of patients with superficial bladder cancer experience recurrence, and 10 to 15% of those patients progress to muscle invasive disease.
Early stage tumors classified in two groups: low grade tumors which are papillary and superficial and high grade tumors either papillary or nonpapillary and often invasive.
Superficial tumors are stages Ta, Tis, and T1 account for 75-85% of cancers while the remaining 15-25% are invasive T2, T3, and T4 or metastatic at the time of presentation.
20% patients present with invasive disease and 5% with metastases.
15-20% of patients will progress from non- invasive to -invasive disease.
Patients with primary tumors that are non- invasive going on to cystectomy are frequently upstaged to invasive tumor in as many as 30-40% of cases.
FDG-PET scan in muscle-invasive bladder cancer reveals nearly 20% of patients are upstaged and the observed upstaging is clinically relevant, with the therapeutic strategy changed in a significant proportion of these patients.
Patients with pT1 disease at cystectomy have lymph node metastases on the order of 10 to 18% of cases.
High-grade T1 bladder cancers have a high propensity to progress and become lethal.
Muscle invasive disease associated with 45% survival at 5 years.
50% of patients with invasive lesions succumb to the disease.
Approximately 30% of T1 patients treated by transurethral resection of the bladder (TURB) develop invasive or higher stage bladder cancer.
Non-muscle invasive bladder cancer is characterized by frequent recurrence and high morbidity but a low risk of mortality.
Muscle invasive bladder cancer is potentially lethal in approximately 50% of cases overall.
Papillary non muscle invasive develops via epithelial hyperplasia and recruitment of a branching vasculature.
Muscle invasive bladder cancer is proposed to develop via flat dysplasia and carcinoma in-situ.
Non-invasive tumors Ta, tumor in-situ, or T1 are treated with transurethral resection with or without intravesical instillation therapy, with recurrence rate as high as 50-70% and an average risk of 10-20% have progression to invasive disease.
Approximately 20% of non- invasive tumors are cured by surgery, while 50-70% recur one or more times without progressing to invasive disease and 10-30% progress to invasive and potentially lethal disease.
5-year all cause survival rate in patients with invasion is 60-75%.
Time to progression to invasion is unpredictable.
Low grade noninvasive papillary tumors are characterized by mutations in HRAS and fibroblast growth factor receptor 3 (FGFR3).
Urothelial bladder cancers that are invasive are characterized by mutations or deletions in tumor suppressor genes, such as TP53, R b, and PTEN, leading to genomic instability and more aggressive phenotypic behavior.
Lesions usually develop in the bladder neck and lateral walls.
Five times higher incidence of bladder cancer in hairdressers and a three times higher risk in women who use dark hair dye for more than 15 years.
The standard treatment for Ta and T1 disease is transurethral resection but 40-80% of patient’s tumors recur within 12 months.
Transurethral resection with intravesical BCG provides a significantly better prophylaxis of tumor recurrence in Ta and T1 bladder cancer than transurethral resection alone.
Photodynamic therapy (PDD) improves tumour detection and reduces residual disease after TURBT compared with white light cystoscopy, and shown to improve RFS but not progression to more advanced disease.
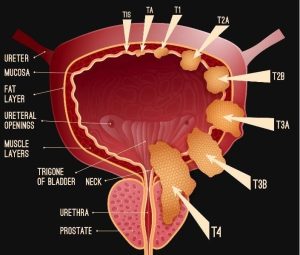
Superficial disease Ta,T1, infiltrating disease T2-4, metastatic disease N+ or M+.
T1 is defined as tumor invading connective tissue under the epithelium.
20-30% of tumors present as invasive carcinomas equal or greater than T1 stage.
Approximately 30% of T1 patients treated by transurethral resection of the bladder (TURB) develop invasive or higher stage bladder cancer.
Histologic examination of specimens obtained by TURB suggest that 5-year progression free survival for patients with depth of invasion of less than 1.5 mm is 93%, compared with 67% for those with depth of invasion of equal or greater than 1.5 mm.
Studies of radical cystectomy for patients with pT1N0 disease have estimated five-year disease specific survival rates from 80-90%.
Management of nonmetastatic muscle invasive bladder cancer requires a multi modality approach: neoadjuvant chemo therapy with cisplatin based regimens followed by radical cystectomy with pelvic lymph node dissection is the preferred treatment for clinical T2-T4a disease.
No standard adjuvant systemic therapies improve outcomes in patients with pathological evidence of residual disease after neoadjuvant cisplatin-based chemotherapy.
Perioperative mortality can reach 14.8% in elderly patients.
5-year survival for patients with cancer extending into perivesical fat when lymph node involvement is not present is 22%.
Almost no reports of bladder cancer as an incidental finding at autopsy studies indicating almost all bladder cancers are clinically sufficient to be diagnosed during life.
Patients who are not eligible for cystectomy undergo radiotherapy with or without chemotherapy.
The recommended primary treatment for stage IIIB disease includes systemic therapy to downstage, followed by tumor reassessment 2 to 3 months after treatment, or concurrent chemoradiotherapy.
Organ sparing multi modality approaches entails maximum transurethral resection, external beam radiotherapy with concurrent radiosensitizing chemotherapy, and often either neoadjuvant were adjuvant chemo therapy-trimodal therapy (TMT).
No prospective randomized trials have established efficacy of TMT versus radical cystectomy but it is included in the guidelines of the American urological association as an alternative in well select the patients with muscle invasive bladder cancer.
Patients who undergo systemic therapy and experience progression are treated using recommendations for metastatic disease, whereas complete responses are treated with subsequent consolidation cystectomy or chemoradiotherapy, or observation.
Patients experiencing a partial response undergo cystectomy or chemoradiotherapy or are treated using guidelines for metastatic disease.
Presently, immune checkpoint blockade inhibitors are approved for metastatic urothelial cancers: PD-L1 inhibitors atezolizumab, durvalumab, Avelumab, and PD-1 nivolumab and pembrolizumab.
Atezolizumab and pembrolizumab as a preferred first-line treatment option for cisplatin-ineligible patients whose tumors express programmed death ligand 1 (PD-L1), or who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 expression.
The new recommendations for first-line systemic therapy in cisplatin-eligible patients with locally advanced or metastatic bladder cancer include gemcitabine and cisplatin or accelerated methotrexate, vinblastine, doxorubicin, and cisplatin with growth factor support.
In the KEYNOTE – 361 study a randomized trial of Pembrolizumab alone or combined with Chemotherapy versus Chemotherapy as first line therapy for advance urothelial carcinoma did not significantly improve efficacy and should not be adopted for treatment of advanced urothelial carcinoma.
Post-platinum–based chemotherapy, subsequent systemic therapy recommendations for patients with locally advanced or metastatic disease include immune checkpoint inhibition with pembrolizumab or atezolizumab, nivolumab, durvalumab, or avelumab:presently the standard of care.
Following immune checkpoint inhibitors, subsequent systemic therapy recommendations now include gemcitabine/carboplatin for cisplatin-ineligible, chemotherapy-naïve patients, or cisplatin and gemcitabine.MRI and CT scans to evaluate lymph node metastases in the pelvis have low sensitivity of 0-30% for bladder cancer.
In a series of 507 cystectomy patients with a negative preoperative CT scan and a median of 22 lymph nodes removed a 24% of patients had nodal metastases (Madersbacher S).
Tis-as many as 20% with diffuse involvement have invasion and about 10% with focal involvement have occult regional lymph node metastases.
70% present initially as superficial tumors and their risk of progression to invasion is low at 5-10%.
Low grade tumors rarely progress (approximately 2% progression rate) and remain confined the (mucosa stage Ta).
On going surveillance is required after diagnosis and treatment of early stage disease with repeated cystoscopy and urinary cytology and regular local resections are often needed to control recurrent disease.
Transurethral resection with surveillance cystoscopy is sufficient treatment for most low grade noninvasive tumors.
While most patients with low-grade noninvasive tumors will recur within five years that they will rarely invade or result in death.
A single administration of perioperative intravesical chemotherapy reduces the risk of recurrence in non- invasive disease treated with transurethral resection.
No single agent given in the perioperative period by intravesical administration is superior in preventing recurrence of low grade noninvasive tumors.
Perioperative intravesical chemotherapy is associated with a 10 to 15% reduction in recurrence rates in low grade non- invasive disease.
High risk patients with non- invasive bladder cancer include patients with high-grade disease, and patients with carcinoma in situ and include patients with lesions greater than 3 cm, multifocal tumors, lamina propria invasion and with recurrence within two years.
In patients with high risk non- invasive bladder disease intervention with intravesical therapy leads to response rates up to 85%, with recurrence rates of more than 50% within the first year and 90% by five years.
In patients with high-risk non- invasive bladder cancers have a 50% progression to invasive disease despite intravesi therapy.
Flat or diffusely infiltrating bladder tumors have a twofold increase progression risk compared to papillary bladder lesions.
Most deaths due to bladder cancer may be attributed to invasive, high-grade stages T2 or greater disease.
At initial diagnosis almost 50% of patients with high-grade bladder cancer have invasive disease.
30% of those patients with initial low-grade tumor have a higher grade recurrence.
BCG can effectively treat patients with residual superficial cancers with a 60% response rate.
Intravesical bacillus Calmette-Guerin is the main treatment for carcinoma in situ or high grade superficial bladder carcinoma.
The FDA approved pembrolizumab for the treatment of patients with bacillus Calmette-Guérin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for, or have elected not to undergo, cystectomy.
KEYNOTE-057 trial which enrolled 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors.
Patients received 200 mg of pembrolizumab every three weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC, or progressive disease for up to 24 months.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41%, and median response duration was 16.2 months.
Nearly half of responding patients (46%) experienced a complete response lasting at least 12 months.
The most common adverse reactions (incidence ≥10%) in patients who received pembrolizumab in KEYNOTE-057 were fatigue, diarrhea, rash, pruritus, musculoskeletal pain, hematuria, cough, arthralgia, nausea, constipation, urinary tract infection, peripheral edema, hypothyroidism and nasopharyngitis.
In cystectomy the pelvic lymph node dissection is the standard treatment for invasive bladder carcinoma with 5-year survival rates for all stages 48-66%, indicating that the majority of patients with high grade invasive disease die of metastases within 3 years.
In cystectomy lymph node dissection involved removal of all tissue within the boundaries of the genitofemoral nerve laterally, the bladder medially, the circumflex iliac vein inferiorly (known as the lymph node of Cloquet), the bifurcation of the common iliac artery superiorly, and the hypogastric blood vessels posteriorly.
In cystectomy lymph nodes or also removed from around the external iliac, internal iliac, obturator blood vessels, and extended lymph node dissection is carried up to the aortic bifurcation and presacral lymph nodes.
Lymph node density, that is, the number of positive nodes as a percentage of total lymph nodes removed, reflects the quality of nodal dissection and tumor burden (Stein JP ET AL).
Lymph node density performs better than the nodal, N, status of the TNM staging in risk stratification, and suggests extended lymph node dissection can be curative in patients with metastases or micrometastases (Karl A et al).
Pelvic lymph node dissection is an essential component of the surgical management of urothelial carcinoma of the bladder, allowing for postoperative risk stratification and improves identification of candidates for adjuvant therapy.
Studies suggest that lymph node dissection improves disease specific and overall survival.
The examination of at least 9 lymph nodes ensure adequate assessment of bladder cancer following cystectomy for proper staging.
Survival is closely related to pathologic stage, but clinical staging diverges from pathologic staging in about 50% of cases.
Cystectomy primary treatment for localized or regionally advanced bladder cancer, as well as high risk superficial tumors that are resistant to intravesical therapy.
Cysstectomy involves removal of the bladder and seminal vesicles in men, and removal of the bladder, uterus, fallopian tubes, ovaries, and a segment of the anterior vaginal wall in females.
Following cystectomy colon is utilized for reconstruction to store and empty urine.
Cystectomy last between 4-10 hours, an in the hospital stay ranging between 5-10 days, and is associated with postoperative complications including atelectasis, ileus, and wound infections.
Lymph node dissection is an essential component of cystectomy.
Patients with orthotopic neobladder following cystectomy have higher rates of complications including incontinence bowel and sexual function impairment is then patients who can have a bladder preserved (Gilbert SM).
5 year overall survival rates for patients treated with cystectomy or a more conservative approach with similar clinical staging is about 50%.
Of patients that present with invasive disease 50% have distant metastases within 2 years, and 60% die within 5 years.
For muscle invasive disease:
To determine whether a patient is a candidate for cystectomy, they should undergo abdominal and pelvic CT or MRI imaging, chest imaging, and a bone scan if bone metastasis is clinically suspected.
Primary treatment is neoadjuvant cisplatin-based combination chemotherapy and subsequent radical cystectomy or partial cystectomy in highly-selected patients with solitary lesions and no Tis, or cystectomy alone if patients are ineligible for cisplatin-based chemotherapy.
Patients who fail neoadjuvant treatment have worse clinical outcome compared to patients who have primary disease.
N1 and N2 disease had been prognostically classified as stage IV previously, but N1 is now in the IIIA group, and N2 and N3 are classified as IIIB.
Patients with stage IIIA (cT3, N0; cT4a, N0; and Ct1-T4a, N1) bladder cancers are to undergo abdominal and pelvic CT or MRI, chest imaging, and bone scan (if bone metastasis is suspected), and are then recommended to undergo neoadjuvant cisplatin-based chemotherapy plus radical cystectomy, or concurrent chemoradiotherapy.
Typically 30-80% of patients who have a bladder cancer have another lesion within 3 years after treatment of the initial tumor.
Bladder cancer-superficial tumors treated transurethrally 50%-75% recur.
5-year survival for patients with positive nodal disease at cystectomy ranges from 5-30%.
Median survival for patients with unresectable disease 7-20 months.
For locally advanced disease chemotherapy is the standard treatment and despite 50% response rate the cure rate is less than 10%.
Elderly patients treated with cystectomy have higher pathological stage and poorer relapse-free survival than younger patients
Telomerase activity detected in almost all superficial urothelial cell cancers but not in healthy urothelial cells.
Cisplatin-based neoadjuvant chemotherapy improved survival in patients with localized muscle invasive bladder cancer.
Methotrexate, vinblastine, doxorubicin, and cyclophosphamide (MVAC) vs gemcitabine and cisplatin (GC) in advanced disease: 405 patients similar response rates and survival 14.8 months vs 13.8 months, respectively (von der Masse H et al).
Second line chemotherapy for metastatic disease associated with
limited efficacy and a median survival of approimtely 7 months.
Gemcitabine/cisplatin rate of down staging in neoadjuvant therapy is comparable to that of MVAC.
Approximately 38% of patients receiving neoadjuvant therapy for localized invasive have a complete pathological response at cystectomy, and the remaining patients have either residual disease or progression.
In the above study GC better safety profile and tolerability, and is presently standard treatment for metastatic disease (2010).
Neoadjuvant chemotherapy in invasive bladder cancer-comparing local treatment alone vs local treatment alone preceded by three cyles of neoadjuvant cisplatin, methotrexate and vinblastine: 16% reduction in the risk of death, correspondinig to an increase in 10 year survival from 30% to 36% (International Trialists Phase III trial Assessing Neoadjuvant Chemotherapy for Invasive Bladder Cancer).
Patients who receive pathologic response to neoadjuvant chemotherapy of either a complete response or eradication of muscle invasive disease have an improved prognosis compared with those with residual muscle invasive or greater disease.
Five-year survival is 35% of patients with regional disease, and 4.8% with distant metastatic disease.
Neoadjuvant chemotherapy with MVAC and gemcitabine / Platinum have absolute survival benefit of 14% after cystectomy at 5 years.
Gemcitabine/Platinum is preferred as neoadjuvant therapy because it is less toxic than MVAC.
Neoadjuvant chemotherapy followed by definitive local therapy is presently the state of the art for deeply invasive bladder cancer, compared with cystectomy or radiotherapy alone.
Neoadjuvant platinum-based combination chemotherapy followed by radical cystectomy with pelvic lymph node dissection is an accepted standard of care for clinical T2 – T4a muscle-invasive bladder cancer.
Cisplatin, methotrexate, vinblastine, doxirubicin neoadjuvant regimens have a reproducible survival benefit, with an incremental 5-7% absolute survival.
In a phase 3 trial of 360 patients with invasive bladder cancer ranomized to radiotherapy with or without synchronous chemotherapy (5FU+Mitomycin): combination management improved locoregional control of bladder cancer without increasing adverse events-5 year survival in the chemoradiotherapy group was 48% vs. 35% in the radiotherapy alone group. (James ND et al).
In the above study combination treatment was associated with a relative reduction of 33% in the risk of locoregional recurrence with a reduction of almot 50% in invasive recurrence.
A study of 3,200 adults age 66 years and older diagnosed with clinical stage T2 to T4a bladder cancer between 2002 and 2011 underwent trimodal therapy of neoadjuvant chemotherapy and radiotherapy and 687 who underwent radical cystectomy alone: Patients who underwent trimodal therapy had significantly decreased overall survival (WilliamsS)
No proof adjuvant chemotherapy after surgery or radiation improves survival vs salvage chemotherapy at relapse.
Patients with muscle-invasive transitional cell carcinoma (TCC) of the bladder have poor survival rates of ~50% at 5 years, after cystectomy.
Postoperative adjuvant chemotherapy allows pathologic staging prior to systemic chemotherapy, limiting toxicity to those patients who are most likely to benefit.
Postoperative adjuvant chemotherapy suggested a 9% improvement in absolute survival at 3 years and a 25% relative reduction in death, but analysis is limitedh by small numbers..
Leow, JJ and colleagues, published recently in European Urology, is a systematic review of the literature and a literature-based meta-analysis of adjuvant chemotherapy trials:
The overall survival results are of borderline significance.
Results from the phase III EORTC adjuvant trial, the largest randomized trial reported of adjuvant chemotherapy for muscle-invasive bladder cancer: Immediate adjuvant cisplatin-based combination chemotherapy after radical cystectomy led to a statistically significant improvement in progression-free survival and a non-significant reduction of 22% in the risk of death.
In the above study, analysis suggested that immediate chemotherapy prolonged survival in node-negative patients, but that there is greater absolute benefit in patients with nodal involvement.
For metastatic disease, gentamicin/cisplatinum, and dose dense MVAC are commonly used.
PD-1 inhibitors @20% response rate in refractory patients.
Pembrolizumab approved for treatment of patients with locally advanced or metastatic disease who have had progression during or following platinum containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum containing drugs.
In the Keynote-045 study Pembrolizumab was compared to chemotherapy in patients with metastatic urothelial cancer after platinum based chemotherapy progression: Pembrolizumab median overall survival was 10.3 months versus chemotherapy 7.4 months.
The overall response rate was 21% with Pembrolizumab versus 11% for cytotoxic therapy, and the 12 month overall survival rate was 44% versus 30.7% favoring Pembrolizumab over chemotherapy.
Atezolizumab approved for cisplatinum ineligible and failed patients with locally advanced or metastatic urothelial cancer.
IMvigor 130 study randomized patients with metastatic urothelial tumors who were untreated and found that atezolizumab added to chemotherapy was associated with a significantly longer median disease free survival. (11.8 months vs. 6.3 months) than chemotherapy alone.
Currently patients are candidate for immunotherapy based on their performance status and prognostic respect is, regardless of PD-L1 status.
Ipilimubab broadens PD-1 activity in urothelial cancers.
KEYNOTE-361 ant IMVigor 130 trials compared PD-L1 low expression patients to platinum based chemotherapy and showed decreased survival.
Ipilimubab plus nivolumab associated with 38.5% objective response rate in metastatic urothelial cancer.
Enfortumab plus pembrolizumab doubled the overall survival of patients versus chemotherapy in metastatic bladder cancer.
Enfortumab plus Pembrolizumab is first line care for metastatic urothelial cancer (2024).
The FDA approved pembrolizumab for the treatment of patients with bacillus Calmette-Guérin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for, or have elected not to undergo, cystectomy.
KEYNOTE-057 trial which enrolled 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors.
Patients received 200 mg of pembrolizumab every three weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC, or progressive disease for up to 24 months.
The complete response rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41%, and median response duration was 16.2 months.
Nearly half of responding patients (46%) experienced a complete response lasting at least 12 months.
