 Most common indication for lung transplantation is emphysema.
Most common indication for lung transplantation is emphysema.
More than 2500 lung transplantations performed annually worldwide, and about 1500 are done in the US.
Candidates for a lung transplant is defined as an estimated risk of dying from lung disease within two years of greater than 50% and, with the assumption of adequate allograft function, likelihood of living five years after transport, transplantation of greater than 80%.
Lung transplantation, or pulmonary transplantation, is a surgical procedure in which one or both lungs are replaced by lungs from a donor.
Bilateral, sequential lung transplantation is the most common procedure for all lung disease indications.
The use of single lung transplantation has decreased, although it remains a valuable option with similar outcomes and fewer postoperative complications, especially in the elderly.
The use of heart-lung transplantation has also decreased overtime and is reserved for patients with both end-stage heart and lung failure that cannot be corrected by bilateral lung transplantation with cardiac repair.
Bilateral thoracotomy with transverse sternotomy (clamshell incision) allows access to both lungs, mediastinal structures and the heart for central cannulation for extracorporeal life support.
A median sternotomy anatomy with venoarterial ECMO or cardiopulmonary bypass allows better decompression of the heart, but requires more anticoagulation, whereas a bilateral anterior sternal sparing thoracotomy results in fewer external healing complications.
Donor lungs can be from a living or deceased donor.
A living donor can only donate one lung lobe.
Some lung diseases may only need to receive a single lung, while other lung diseases such as cystic fibrosis need to receive two lungs.
Lung transplants can also extend life expectancy and enhance the quality of life for those with end stage pulmonary disease.
Lung transplantation is a measure of last resort for patients with end-stage lung disease who have exhausted all other available treatments without improvement.
The most common indications for lung transplantation include: pulmonary fibrosis, COPD, pulmonary vascular disease, and cystic fibrosis.
Conditions that may make such surgery necessary:
chronic obstructive pulmonary disease (COPD), including emphysema;
idiopathic pulmonary fibrosis
cystic fibrosis
idiopathic pulmonary hypertension
alpha 1-antitrypsin deficiency
replacing previously transplanted lungs that have since failed
bronchiectasis and sarcoidosis
Contraindications:
Certain conditions may make a person a poor candidate for lung transplantation:
Concurrent chronic illness: congestive heart failure, kidney disease, liver disease
Current infections, including HIV and hepatitis
However, hepatitis C patients are both being transplanted and are also being used as donors if the recipient is hepatitis C positive.
Similarly, select HIV-infected individuals have received lung transplants after being evaluated on a case-by-case basis.
Current or recent cancer
Current use of alcohol, tobacco or illegal drugs
Age
Psychiatric conditions
History of noncompliance with medical instructions
Requirements for potential donors:
Requirements for potential lung donors:
Size match: donated lung or lungs must be large enough to adequately oxygenate the patient, but small enough to fit within the recipient’s chest cavity
Blood type
Requirements for potential recipients:
End-stage lung disease
Has exhausted other available therapies without success
No other chronic medical conditions of heart, kidney, liver.
No current infections or recent cancer.
Some patients with lung cancer or other cancers, may be allowed.
There has been a steady increase in the age of lung recipients, with 34% of recipients being older than age 65 in the US in 2021, with emphasis on physiologic over chronological age.
Assessments of coronary and cerebral arteries, kidney function, bone health, esophageal function, psychosocial capacity, and social support are important evaluations.
In certain cases where pre-existing infection is unavoidable, as with many patients with cystic fibrosis.
Patients are evaluated for frailty, sarcopenia and degree of resilience to predict recoverability.
Patients who have received mechanical ventilation, and even extracorporal life support are in increasingly common transplant participants.
No HIV or hepatitis, although some recipients with the same type of hepatitis as the donor can receive a lung, and individuals with HIV who can be stabilized and can have a low HIV viral load may be eligible.
No alcohol, smoking, or drug abuse
Marked undernourishment or obesity are both associated with increased mortality.
Acceptable psychological profile
Has a social support system
Able to comply with post-transplant regimen.
Blood typing; the recipient’s blood type must match the donor’s, due to antigens that are present on donated lungs.
A mismatch of blood type can lead to a strong response by the immune system and subsequent rejection of the transplanted organs.
Tissue typing; ideally should match as closely as possible between the donor and the recipient, but the desire to find a highly compatible donor organ must be balanced against the patient’s immediacy of need.
Chest X-ray – PA & LAT, to verify the size of the lungs and the chest cavity
Pulmonary function tests
CT Scan=Thoracic & Abdominal
Bone mineral density scan
MUGA scan
Cardiac stress test (Dobutamine/Thallium scan)
Ventilation/perfusion (V/Q) scan
Electrocardiogram
Cardiac catheterization
Echocardiogram
Lung allocation score: prospective lung recipients of age of 12 and older are assigned a lung allocation score or LAS, which takes into account various measures of the patient’s health.
The new system allocates donated lungs according to the immediacy of need rather than how long a patient has been on the transplant list.
Patients who are under the age of 12 are given priority.
The length of time spent on the list is also the deciding factor when multiple patients have the same lung allocation score.
Types of lung transplant:
A lobe transplant is a surgery in which part of a living or deceased donor’s lung is removed and used to replace the recipient’s diseased lung.
Donors should be able to maintain a normal quality of life despite the reduction in lung volume.
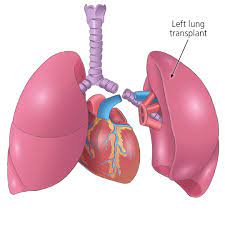
Many patients can be helped by the transplantation of a single healthy lung,from a donor who has been pronounced brain-dead.
Patients may require both lungs to be replaced, especially for people with cystic fibrosis, due to the bacterial colonization commonly found within such patients’ lungs.
If only one lung were transplanted, bacteria in the native lung could potentially infect the newly transplanted organ.
Some respiratory patients may also have cardiac disease which would necessitate a heart transplant: a domino transplant.
A single lung transplant takes about four to eight hours, while a double lung transplant takes about six to twelve hours to complete.
A history of prior chest surgery may complicate the procedure and require additional time.
In single-lung transplants, the lung with the worse pulmonary function is chosen for replacement.
The right lung is usually favored for removal because it avoids having to maneuver around the heart, as would be required for excision of the left lung.
In a singular lung transplant the lung is collapsed, the blood vessels in the lung tied off, and the lung removed at the bronchial tube.
The donor lung is placed, the blood vessels and bronchial tube reattached, and the lung reinflated.
A double-lung transplant, also known as a bilateral transplant, can be done either sequentially, en bloc, or simultaneously.
Sequential is more common than en bloc: equivalent to having two separate single-lung transplants done.
The transplantation process starts after the donor lungs are inspected and the decision to transplant has been made.
In 10% to 20% of double-lung transplants the patient is hooked up to a heart-lung machine which pumps blood for the body and supplies fresh oxygen.
The average hospital stay following a lung transplant is generally one to three weeks.
Patients are typically required to attend a rehabilitation program for approximately 3 months to regain fitness.
Certain nerve connections to the lungs are cut during the procedure, so transplant recipients cannot feel the urge to cough or feel when their new lungs are becoming congested.
Lung transplantation patients must make conscious efforts to take deep breaths and cough in order to clear secretions from the lungs.
The heart rate response is less quick to exertion due to the cutting of the vagus nerve that would normally help regulate it.
Patient may notice a change in their voice due to potential damage to the nerves that coordinate the vocal cords.
Exercise enhances muscle strength, increase bone mineral density as well as improvements in 6 minute walking time.
Immunosuppressant drugs are required to prevent transplant rejection.
Which make recipients vulnerable to infections.
Complications of lung transplant: bleeding, infection, rejection, failure to properly heal and function, post-transplant lymphoproliferative disorder, gastrointestinal inflammation and ulceration of the stomach and esophagus.
Vascular complications at the level of the pulmonary artery or left atrial anastamoses including kinking, stenosis, thrombosis, and infarction are rare, but may result in graft loss or death.
Partial dehiscence of the airway anastamoses effects of to 10% of lung transplant recipients.
Acute airway ischemia and secondary infection are additional risk factors.
Other complications include airway stenosis, malacia defined this dynamic collapse of the airway to less than 50% in diameter are additional management complications that can include mucus retention, recurrent infection, and breathlessness with wheeze and strider.
Transplant rejection may occur both immediately after the surgery and continuing throughout the patient’s life.
Primary graph dysfunction after transplant is an acute lung injury characterized by pulmonary edema and hypoxemia.
Severe primary graph dysfunction occurs in 15 to 25% of transplants and has a major effect on the length of stay in the ICU and a 90 day and one year mortality.
Chronic lung allograft dysfunction (CLAD) is more likely to develop in survivors of primary graft dysfunction who have higher long-term mortality.
Increase risk of primary graft dysfunction occurs with greater recipient frequency of pre-transplantation, lung fibrosis, and pulmonary hypertension.
ECMO is commonly used in patients with severe primary graft dysfunction.
Ventilation strategy after lung transplant is to reduce lung injury through lung protective ventilation.
Infections are common in the early post transplant period, and the use of prophylactic antibiotics is routine.
Bacterial, fungal, and viral infections are a major cause of illness and death.
Primary infection with or reactivation of latent cytomegalic virus is common and leads to systemic illness, graft injury, and increased risk of chronic lung allograft dysfunction.
Acute kidney injury secondary to effects of surgery and critical illness, nephrotoxic effects of immunosuppression and antimicrobials contributes to early complications and can lead to chronic kidney disease.
Acute cellular rejection affects 20 to 50% of recipients in the first year and is driven by T cell infiltration into the allograft.
Acute cellular rejection is diagnosed on the base of trans bronchial biopsies.
In most cases, acute cellular rejection resolves with steroid augmentation.
Additional management for acute rejection includes anti-thymocyte globulin, total lymphoid radiation, or extra corporal photophoresis.
Antibody mediated rejection with antibodies against donor HLA or non-HLA antigens can also occur in isolation or alongside acute cellular rejection.
Signs of rejection:
fever, flu-like symptoms, including chills, dizziness, nausea, general
feeling of illness, night sweats;
increased difficulty in breathing;
worsening pulmonary test results;
increased chest pain or tenderness;
increase or decrease in body weight of more than two kilograms in a 24-hour period.
The immunosuppressive regimen is begun just before or after surgery, and usually includes cyclosporine, azathioprine, antithymocyte globulin and corticosteroids.
Episodes of rejection may reoccur throughout a patient’s life, the exact choices and dosages of immunosuppressants may have to be modified over time.
Other agents include tacrolimus and mycophenolate mofetil.
Chronic rejection, meaning repeated bouts of rejection symptoms beyond the first year after the transplant surgery, occurs in approximately 50% of patients.
Chronic lung allograft dysfunction (CLAD) is a result of innate in adaptive alloimmune responses in the lung allograft leading to dysregulated repair and fibrosis in airways and parenchymal compartments.
Such chronic rejection presents itself as bronchiolitis obliterans, or less frequently, atherosclerosis.
The lung transplant survival rate one year after transplant is as high as 88 percent.
After 3 years, the lung transplant survival rate is 73 percent.
The 5-year lung transplant survival rate is 60 percent.
The ten-team survival rate is about 28%.
Lung allograft health is monitored with longitudinal spirometry: a fall in FEV1 greater than 10% triggers investigation.
Transplanted lungs typically last three to five years before showing signs of failure.
A study of nearly 10,000 lung transplant recipients demonstrated significantly improved long-term survival using sirolimus + tacrolimus (median survival 8.9 years) instead of mycophenolate mofetil + tacrolimus (median survival 7.1 years) for immunosuppressive therapy starting at one year after transplant. Immunosuppression strategies include induction therapy that deplete or inhibit lymphocytes in the peritransplantation period in many centers.
Induction therapy when used includes basilliximab a monocolonal IL-2 receptorantagonist and other drugs include alemtuxumab anti-thymocyte globulin.
Maintenance immunosuppression regimens comprising, a calcium inhibitor, a low-dose glucocorticoid and a cell cycle inhibitor are used almost universally.
Additional immunosuppressant agents such as mammalian target of rapamycin,, tacrolimus/cyclosporine, everolimus/mycophenolate and belacept are additional agents.
Cancer is more common in post lung transplantation patients than in the general population and is frequently associated with a worse prognosis accounting for up to 17% of deaths.
Lung cancer and post transplantation lymphoproliferative disease are the most common causes of cancer related mortality and lung transplant patients.
Non-melanoma skin cancers are the most common cancers among transplant recipients.
Median survival after lung transplantation is about 6.7 years with little progress in long-term outcomes over the last decades.
