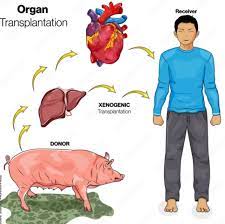
 Xenotransplantation is the transplantation of living cells, tissues or organs from one species to another.
Xenotransplantation is the transplantation of living cells, tissues or organs from one species to another.
Such cells, tissues or organs are called xenografts.
This is is contrast with allotransplantation from other individual of same species, syngeneic transplantation or isotransplantation grafts between two genetically identical individuals of the same species and autotransplantation, from one part of the body to another in the same person.
Xenotransplantation is an artificial method of creating an animal-human chimera, that is, a human with a subset of animal cells.
Human xenotransplantation offers a potential treatment for end-stage organ failure.
Safety in clinical xenotransportation requires an assessment of infectious risks by transplant of pathogens to immunosuppressed recipients, and to the public.
However, continuing concern is that many animals, such as pigs, have a shorter lifespan than humans, meaning that their tissues age at a quicker rate.
Pigs have a maximum life span of about 27 years.
Disease transmission, or xenozoonosis, and permanent alteration to the genetic code of animals are also causes for concern.
Animal rights activists object to xenotransplantation on ethical grounds.
Bioprosthetic artificial heart valves are generally pig or bovine-derived, but the cells are killed by glutaraldehyde treatment before insertion, therefore technically not fulfilling the WHO definition of xenotransplantation of being live cells.
A worldwide shortage of organs for clinical implantation causes about 20–35% of patients who need replacement organs to die on the waiting list.
Certain procedures use cells or tissues from other species to treat life-threatening and debilitating illnesses such as cancer, diabetes, liver failure and Parkinson’s disease.
Xenotransplants could save thousands of patients waiting for donated organs.
An animal organ, could be genetically altered with human genes to trick a patient’s immune system into accepting it as a part of its own body.
Pigs currently thought to be the best candidates for organ donation.
The risk of cross-species disease transmission is decreased because of their increased phylogenetic distance from humans.
Pigs have relatively short gestation periods, large litters, and are easy to breed, making them readily available.
They are inexpensive and easy to maintain in pathogen-free facilities, and current gene editing tools are adapted to pigs to combat rejection and potential zoonoses.
Pig organs are anatomically comparable in size, and new infectious agents are less likely since they have been in close contact with humans through domestication for many generations.
Treatments sourced from pigs have proven to be successful such as porcine-derived insulin for patients with diabetes mellitus.
Genetically engineered pigs are becoming the norm, increasing the success rate of the transplant.
Current experiments in xenotransplantation most often use pigs as the donor, and baboons as human models.
Pig organs have been used for kidney and heart transplants into humans.
In the field of regenerative medicine, pancreatogenesis- or nephrogenesis allow experimentation toward the in vivo generation of functional organs using blastocyst complementation to generate transplantable human organs from the patient’s own cells, using livestock animals, to increase quality of life for those with end-stage organ failure.
Xenozoonoses are one of the biggest threats to rejections, as they are xenogenetic infections, introducing microorganisms that lead to the fatal infections and then rejection of the organs.
Xenozoonoses response is more extreme than in allotransplantations, ultimately results in rejection of the xenograft, and can in some cases result in a hyperacute rejection and is currently not completely understood.
There are several types of rejection organ xenografts, these include hyperacute rejection, acute vascular rejection, cellular rejection, and chronic rejection.
A rapid, violent, and hyperacute response comes as a result of antibodies present in the host organism.
These antibodies are known as xenoreactive natural antibodies (XNAs).
Hyperacute rejection is a rapid and violent type of rejection that occurs within minutes to hours from the time of the transplant. It is mediated by the binding of XNAs (xenoreactive natural antibodies) to the donor endothelium, causing activation of the human complement system, which results in endothelial damage, inflammation, thrombosis and necrosis of the transplant.
XNAs are first produced and begin circulating in the blood in neonates, after colonization of the bowel by bacteria with galactose moieties on their cell walls.
Most of these antibodies are the IgM class, but also include IgG, and IgA.
The binding of XNAs initiate complement activation causing a cascade of events leading to: destruction of endothelial cells, platelet degranulation, inflammation, coagulation, fibrin deposition, and hemorrhage.
This results in thrombosis and necrosis of the xenograft.
Acute vascular rejection,
also known as delayed xenoactive rejection, this type of rejection occurs in discordant xenografts within 2 to 3 days, if hyperacute rejection is prevented.
Acute vascular rejection requires de novo protein synthesis and is driven by interactions between the graft endothelial cells and host antibodies, macrophages, and platelets.
The response is characterized by an inflammatory infiltrate of mostly macrophages and natural killer cells, intravascular thrombosis, and fibrinoid necrosis of vessel walls.
Binding of XNAs to the donor endothelium leads to the activation of host macrophages as well as the endothelium itself.
The binding of xenoreactive natural antibodies (XNAs). ultimately leads to the development of a procoagulant state, the secretion of inflammatory cytokines and chemokines, as well as expression of leukocyte adhesion molecules such as E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1).
The use of immunosuppressive drugs are necessary to prevent acute vascular rejection, and include administering a synthetic thrombin inhibitor to modulate thrombogenesis, depletion of anti-galactose antibodies (XNAs) by techniques such as immunoadsorption, to prevent endothelial cell activation, and inhibiting activation of macrophages and NK cells.
If hyperacute and acute vascular rejection are avoided accommodation is possible, the survival of the xenograft despite the presence of circulating XNAs.
The graft is given a break from humoral rejection[50] when the complement cascade is interrupted, circulating antibodies are removed, or their function is changed, or there is a change in the expression of surface antigens on the graft. This allows the xenograft to up-regulate and express protective genes, which aid in resistance to injury, such as heme oxygenase-1 (an enzyme that catalyzes the degradation of heme).
Rejection of the xenograft in hyperacute and acute vascular rejection is due to the response of the humoral immune system by the XNAs.
Cellular rejection is based on cellular immunity, and is mediated by natural killer cells that accumulate in and damage the xenograft and T-lymphocytes which are activated by MHC molecules through both direct and indirect xenorecognition.
Antigen presenting cells from the xenograft present peptides to recipient CD4+ T cells via xenogeneic MHC class II molecules, resulting in the production of interleukin 2 (IL-2).
Indirect xenorecognition involves the presentation of antigens from the xenograft by recipient antigen presenting cells to CD4+ T cells.
Antigens of phagocytosed graft cells can also be presented by the host’s class I MHC molecules to CD8+ T cells.
Cellular rejection in xenografts is expected to be stronger than in allografts due to differences in peptides among different animals: with more antigens potentially recognized as foreign, thus eliciting a greater indirect xenogenic response.
Chronic rejection is slow and progressive, and usually occurs in transplants that survive the initial rejection phases.
Xenografts rarely survive past the initial acute rejection phases.
Fibrosis in the xenograft occurs as a result of immune reactions, cytokines or healing following cellular necrosis in acute rejection.
Perhaps the major cause of chronic rejection is arteriosclerosis.
Lymphocytes, activate macrophages to secrete smooth muscle growth factors, and results in a build up of smooth muscle cells on the vessel walls, causing the hardening and narrowing of vessels within the graft.
Chronic rejection leads to pathologic changes of the organ, requiring that transplants be replaced after so many years.
It is also anticipated that chronic rejection will be more aggressive in xenotransplants as opposed to allotransplants.
Different organ xenotransplants result in different responses in clotting.
Kidney transplants result in a higher degree of coagulopathy, or impaired clotting, than cardiac transplants, whereas liver xenografts result in severe thrombocytopenia, causing recipient death within a few days due to bleeding.
Some porcine transplant cells are able to induce human tissue factor expression, thus stimulating platelet and monocyte aggregation around the xenotransplanted organ, causing severe clotting.
Dfferences in organ size limit the range of potential recipients of xenotransplants.
Xenozoonosis is the transmission of infectious agents between species via xenograft: example of such is the avian influenza, when an influenza A virus was passed from birds to humans.
Xenotransplantation may increase the chance of disease transmission: for 3 reasons: (1) implantation breaches the physical barrier that normally helps to prevent disease transmission,
the recipient of the transplant will be severely immunosuppressed, and (3) human complement regulators (CD46, CD55, and CD59) expressed in serve as virus receptors, and may also help to protect viruses from attack by the complement system.
Viruses carried by pigs include: porcine herpesvirus, rotavirus, parvovirus, and circovirus, porcine endogenous retroviruses
The risks with xenosis are twofold: the individual become infected, but a novel infection could initiate an epidemic in the human population.
Because of this risk, recipients of xenotransplants should be closely monitored for the remainder of their life, and quarantined if they show signs of xenosis.
Baboons and pigs carry many transmittable agents that are harmless in their natural host, but extremely dangerous and deadly in humans.
An obstacle facing xenotransplants is that of the body’s rejection of foreign objects by its immune system.
These antigens are often treated with immunosuppressive drugs that could, in turn, make the patient vulnerable to other infections and actually aid the disease.
Xenotransplantation has future medical benefits, but it also has the serious risk of introducing and spreading the infectious diseases, into the human population.
If a transplantation takes place the recipient must undergo monitoring for the rest of that recipient’s lifetime and waive their right to withdraw.
The reason for requiring lifelong monitoring is due to the risk of acute infections that may occur.
