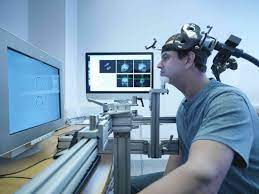
 Transcranial magnetic stimulation (TMS) is a noninvasive form of brain stimulation in which a changing magnetic field is used to induce an electric current at a specific area of the brain through electromagnetic induction.
Transcranial magnetic stimulation (TMS) is a noninvasive form of brain stimulation in which a changing magnetic field is used to induce an electric current at a specific area of the brain through electromagnetic induction.
An electric pulse generator, or stimulator, is connected to a magnetic coil connected to the scalp.
TMS uses electromagnetic induction to generate an electric current across the scalp and skull.
A plastic-enclosed coil of wire is held next to the skull and when activated, produces a varying magnetic field oriented orthogonally to the plane of the coil.
The changing magnetic field then induces an electric current in the brain that activates nearby nerve cells in a manner similar to a current applied superficially at the cortical surface.
The stimulator generates a changing electric current within the coil which creates a varying magnetic field, inducing a current within a region in the brain itself.
Adverse effects of TMS appear rare and include fainting and seizure.
Other potential issues include discomfort, pain, hypomania, cognitive change, hearing loss, and inadvertent current induction in implanted devices such as pacemakers or defibrillators.
A number of reports detail other significant adverse experiences, such as new seizures, psychotic symptoms, and other effects.
A magnetic coil is positioned on the patient’s head.
TMS does not require surgery or electrode implantation.
TMS stimulates cortical tissue without the pain sensations produced in transcranial electrical stimulation.
TMS can be used clinically to measure activity and function of specific brain circuits in humans.
It is most commonly with single or paired magnetic pulses.
The most widely accepted use is in measuring the connection between the primary motor cortex of the central nervous system and the peripheral nervous system to evaluate damage or neurologic insult.
TMS is a neurophysiological method that allows the measurement of the time required for a neural impulse to cross the pyramidal tracts, starting from the cerebral cortex and ending at the anterior horn cells of the cervical, thoracic or lumbar spinal cord: Central Conduction Time (CCT).
TMS can determine whether myelopathy exists and can identify the level of the spinal cord where myelopathy is located.
Repetitive high frequency TMS (rTMS) has shown diagnostic and therapeutic potential with the central nervous system in the fields of neurology and mental health.
Adverse effects generally increase with higher frequency stimulation.
The greatest immediate risk from TMS is fainting, though this is uncommon.
Seizures are rare.
Other adverse effects include short term discomfort, pain, brief episodes of hypomania, cognitive change, hearing loss, impaired working memory, and the induction of electrical currents in implanted devices such as cardiac pacemakers.
The magnetic field is about the same strength as Magnetic resonance imaging (MRI).
Its pulse generally reaches no more than 5 centimeters into the brain unless using a modified coil and technique for deeper stimulation.
TMS is achieved by quickly discharging current from a large capacitor into a coil to produce pulsed magnetic fields between 2 and 3 Tesla in strength.
Directed to a target area in the brain causes a localized electrical current which can then either depolarize or hyperpolarize neurons at that site.
The induced electric field inside the brain tissue causes a change in transmembrane potentials resulting in depolarization or hyperpolarization of neurons, causing them to be more or less excitable, respectively.
Deep TMS can reach up to 6 cm into the brain to stimulate deeper layers of the motor cortex, such as that which controls leg motion.
The brain is irregularly shaped with variable internal density and water content, leading to a nonuniform magnetic field strength and conduction throughout its tissues.
TMS effects are divided based on frequency, duration and intensity of stimulation:
Single or paired pulse TMS causes neurons in the neocortex under the site of stimulation to depolarize and discharge an action potential.
If used in the primary motor cortex, it produces muscle activity referred to as a motor evoked potential (MEP) which can be recorded on electromyography.
If used on the occipital cortex, flashes of light might be perceived by the subject.
In most other areas of the cortex, there is no conscious effect, but behavior may be altered: slower reaction time on a cognitive task, or changes in brain activity may be detected using diagnostic equipment.
Repetitive TMS produces longer-lasting effects which persist past the period of stimulation.
rTMS can increase or decrease the excitability of the corticospinal tract depending on the intensity of stimulation, coil orientation, and frequency.
Low frequency rTMS with a stimulus frequency less than 1 Hz inhibit cortical firing.
A higher stimulus frequency greater than 1 Hz, or high frequency, is believed to provoke cortical firing, which may be due to a change in synaptic efficacy related to long-term potentiation and long-term depression like plasticity.
Most devices use a coil shaped like a figure-eight to deliver a shallow magnetic field that affects more superficial neurons in the brain.
The core material may be either a magnetically inert substrate air core or a solid, ferromagnetically active material solid core.
Solid cores result in more efficient transfer of electrical energy to a magnetic field and reduce energy loss to heat.
Solid cores result in higher volume of therapy protocols without interruption due to overheating.
Differences in coil material and its power supply affect magnetic pulse width and duration.
A number of different types of coils exist, each of which produce different magnetic fields: round, butterfly, four-leaf coil, double-cone coil, Hesed (H-core).
Circular crown and double cone coils allow more widespread activation and a deeper magnetic penetration.
TMS has shown potential therapeutic effect on neurologic conditions such as Alzheimer’s disease, amyotrophic lateral sclerosis, persistent vegetative states, epilepsy, stroke related disability, tinnitus, multiple sclerosis, schizophrenia, and traumatic brain injury.
In Parkinson’s disease, results suggest that low frequency stimulation may have an effect on medication associated dyskinesia, and that high frequency stimulation improves motor function: The most effective treatment protocols appear to involve high frequency stimulation of the motor cortex, particularly on the dominant side, but with more variable results for treatment of the dorsolateral prefrontal cortex.
Cerebellar stimulation has also shown potential for the treatment of levodopa associated dyskinesia.
TMS has shown potential with anxiety disorders, including panic disorder and obsessive–compulsive disorder (OCD).
The most promising areas to target for OCD appear to be the orbitofrontal cortex and the supplementary motor area.
For treatment-resistant major depressive disorder, high-frequency rTMS of the left dorsolateral prefrontal cortex appears effective and low-frequency of the right DLPFC has probable efficacy.
TMS can also be used to map functional connectivity between the cerebellum and other areas of the brain.
There may be neck pain, headache and twitching in the scalp or upper face associated with the intervention.
TMS was found to improve depression significantly in 58 percent of patients and provide complete remission of symptoms in 37 percent of patients.
Other neurological areas for treatment consideration: Alzheimer’s disease, autism, bipolar disorder, epilepsy, chronic pain, major depressive disorder, Parkinson’s disease, post-traumatic stress disorder, schizophrenia, to aid smoking cessation, and obsessive–compulsive disorder.
TMS for obsessive-compulsive disorder (OCD), migraines and to help people stop smoking when standard treatments haven’t worked well.
