Toxoplasmosis
Up to half of the world’s population is infected by toxoplasmosis, but have no symptoms.
Approximately 11% of people in the US are infected.
Some areas of the world more than 60% have toxoplasmosis infection.
Approximately 200,000 cases of congenital toxoplasmosis occur a year.
There is evidence that infection may affect people’s behavior.
Acute toxoplasmosis is often asymptomatic, as it is rare for a human with a fully functioning immune system to develop severe symptoms following infection.
Infection with T. gondii produces no readily observable symptoms in healthy human adults.
Symptoms may appear, and are often influenza-like: swollen lymph nodes, headaches, fever, fatigue, and muscle aches and pains that last for a month or more.
Patients with weakened immune systems are likely to experience symptoms: headache, confusion, poor coordination, seizures, lung problems that may resemble tuberculosis or Pneumocystis jiroveci pneumonia, or blurred vision caused by severe inflammation of the retina.
Severe toxoplasmosis may be seen in young children, immunocompromised people, such as those with HIV/AIDS, chemotherapy, or who have had an organ transplant.
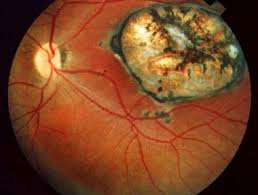
Toxoplasmosis can cause encephalitis or necrotizing retinochoroiditis.
Rarely, infants infected via placental transmission may be born with encephalitis or retinochoroiditis and nasal malformations.
Lymphadenopathy is commonly found in the neck, under the chin, and in the axils and groin.
Adenopathy may persist, and recur for various times independently of antiparasitic treatment.
Adenopathy is usually noted at single sites in adults, but in children, multiple sites may be more common.
Lymphadenopathy will resolve within 1–2 months in 60% of cases: 25% take 2–4 months to return to normal, and 8% take 4–6 months.
About 6% do not return to normal until much later.
In most immunocompetent people, the infection T enters a latent phase, during which only tissue cysts are present.
Such tissue cysts can occur in the retinas, alveolar lining of the lungs,
heart, skeletal muscle, and the central nervous system (CNS), including the brain.
It may cause an acute pulmonary infection that may mimic a Pneumocystis jirovecii infection.
Cysts that form in the CNS upon infection with T. gondii persist for the lifetime of the host.
Most infected infants while in the womb have no symptoms at birth, but may develop symptoms later in life.
Serological studies estimate that 30–50% of the global population has been exposed to and may be chronically infected with latent toxoplasmosis.
The infection rates differ from country to country.
Latent toxoplasmosis is associated with numerous diseases, neural alterations,and subtle gender-dependent behavioral changes in immunocompetent humans.
Latent toxoplasmosis is associated with an increased risk of motor vehicle collisions.
Skin lesions may rarely occur with toxoplasmosis in the acquired form of the disease, including roseola and erythema multiforme-like eruptions, prurigo-like nodules, urticaria, and maculopapular lesions.
Newborns with toxoplasmosis may have macules, ecchymoses, or “blueberry muffin” like lesions.
The tachyzoite form of T. gondii found in all levels of the epidermis, confirms the diagnosis.
Tachyzoites can be identified by electron microscopy or by Giemsa staining tissue.
Tachyzoite form of T. Gondi is responsible for acute infection.
Tachyzoites divide rapidly and spread through the tissues of the body.
Tachyzoites convert into bradyzoites: latent intracellular tissue cysts that form mainly in the muscles and brain.
The formation bradyzoites cysts are part triggered by the hosts immune system.
Bradyzoites are not responsive to antibiotics, and can remain in the tissues for the lifespan of the host.
If bradyzoites convert back into active tachyzoites, the immune system will quickly destroy them in a healthy person.
In immunocompromised individuals, or in fetuses, which lack a developed immune system, the tachyzoites can
cause significant neurological damage.
T. gondii reduces the host’s immune response, enhances its reproduction, and resists damage caused by the host’s immune system.
T. gondii forms a vacuole membrane from the membrane of the host cell that encapsulates the parasite.
This membrane resists the activity of the endolysosomal system, and can control of the host’s mitochondria and endoplasmic reticulum.
T. gondii invades the cell, where they can activate STAT pathways to modulate the expression of cytokines.
T. gondii influences an anti-apoptotic mechanism, allowing the infected host cells to persist and replicate.
T. gondii disrupts pro-apoptosis effector proteins, such as BAX and BAK causing causes conformational changes to the proteins, which prevent the proteins from being transported to various cellular compartments where they initiate apoptosis events.
T. gondii does not cause downregulation of the pro-apoptosis effector proteins.
T. gondii has the ability to initiate autophagy of the host’s cells, decreasing healthy, uninfected cells, and consequently fewer host cells to attack the infected cells.
Infected cells have higher levels of autophagosomes.
T. gondii influence on the host cells is stronger in a weak immune system and dependent on the number of T. gondii per host cell.
Immunocompetent individuals do not normally show severe symptoms.
Severe complications and death can result in immunocompromised individuals.
The T. gondii can change the immune responses, either positive or negative, on the immune response to other pathogenic threats: Helicobacter felis, Leishmania major, or other parasites, such as Nippostrongylus brasiliensis.
Toxoplasmosis transmitted orally when Toxoplasma gondii oocysts or tissue cysts are accidentally eaten.
Oral transmission may occur through:
Ingestion of raw or partly cooked meat, especially pork, lamb, or venison containing Toxoplasma cysts.
Infection prevalence in countries where undercooked meat is traditionally eaten is related to this transmission method.
Tissue cysts may also be ingested from hand-to-mouth contact after handling undercooked meat, or from using knives, utensils, or cutting boards contaminated by raw meat, and by ingestion of unwashed fruit or vegetables that have been in contact with contaminated soil containing infected cat feces, the
ingestion of cat feces containing oocysts.
Transmission can occur through hand-to-mouth contact following gardening, cleaning a cat’s litter box, contact with children’s sandpits; the parasite can survive in the environment for months.
Congenital transmission from mother to fetus can also occur.
Transmission may also occur during the solid organ transplant process or hematogenous stem cell transplants.
Transmission by ingestion of untreated, unfiltered water through direct consumption or utilization of water for food preparation.
Ingestion of unpasteurized milk and milk products, particularly goat’s milk can cause toxoplasmosis transmission, as can ingestion of raw seafood.
Cats excrete the T. gondii pathogen in their feces for a number of weeks after contracting the disease.
Cats generally acquire the infection by eating an infected intermediate host that could include mammals, like rodents, or birds.
Cat oocyst shedding usually starts from the third day after ingestion of infected intermediate hosts, and may continue for weeks.
The cat oocysts are not infective when excreted: After about a day, the oocyst undergoes sporulation and becomes potentially pathogenic.
In addition to cats, birds and mammals including human beings are also intermediate hosts of the parasite and are involved in the transmission process.
Toxoplasmosis may also be transmitted through solid organ transplants:
seronegative recipients who receive organs from recently infected Toxoplasma-seropositive donors are at risk.
In addition, organ recipients withlatent toxoplasmosis are at risk of the disease reactivating due to the immunosuppression occurring during solid organ transplant
Hematogenous stem cell transplant patients are at higher risk of infection due to prolonged periods of immunosuppression.
Heart and lung transplants provide the highest risk for toxoplasmosis infection due to the striated muscle making up the heart, as it can contain cysts.
In congenital toxoplasmosis the unborn fetus is infected via the placenta.
Congenital toxoplasmosis is associated with fetal death, miscarriage, and in infants, with neurologic deficits, neurocognitive deficits, and chorioretinitis.
Pregnant women are not routinely screened for toxoplasmosis in most countries, for reasons of cost and the high number of false positives.
Postnatal or neonatal screening is preferred.
Pregnant women should avoid handling raw meat, drinking raw milk, and not eat raw or undercooked meat regardless of type, avoid exposure to cat feces, and refrain from gardening, or at least wear gloves when so engaged.
Cats get infected in the first six months of their life, when they shed oocysts for a 1–2 weeks.
Oocysts get buried in the soil, sporulate and remain infectious for periods ranging from several months to more than a year.
Living in a household with a cat is not a significant risk factor for T. gondii infection.
Living with several kittens has some significance for toxoplasmosis.
Women with high levels of toxoplasmosis antibodies are significantly more likely to have baby boys than baby girls: women infected with T. gondii have up to a 72% chance of having a boy.
Diagnosis of toxoplasmosis is made by biological, serological, histological, or molecular methods, or by some combination of the above.
CNS Toxoplasmosis can be difficult to distinguish from primary central nervous system lymphoma.
T. gondii may also be detected in blood, amniotic fluid, or cerebrospinal fluid by using polymerase chain reaction.
Serological testing can detect T. gondii antibodies in blood with multiple techniques.
IgG antibodies usually appear within a week or two of infection.
IgG antibodies peak within 1-2 months, then decline at various rates.
Toxoplasma IgG antibodies generally persist for life.
IgM antibodies can be used to detect acute infection but generally not chronic infection.
The IgM antibodies appear sooner after infection than the IgG antibodies and disappear faster than IgG antibodies after recovery.
T. gondii-specific IgM antibodies can first be detected approximately a week after acquiring primary infection and decrease within one to six months.
But 25% of those infected are negative for T. gondii-specific IgM within seven months.
IgM may be detectable months or years after infection, during the chronic phase, creating false positives results for acute infection.
Diagnosis of congenital toxoplasmosis findings include: prenatal diagnosis based on testing of amniotic fluid and ultrasound examinations; molecular testing of placenta and cord blood and comparative mother-child serologic tests and a clinical examination at birth.
The diagnosis in early childhood is based on neurologic and ophthalmologic examinations and a serologic survey during the first year of life.
During pregnancy, serological testing is recommended.
PCR-based techniques have been developed to diagnose toxoplasmosis using clinical specimens that include amniotic fluid, blood, cerebrospinal fluid, and tissue biopsy.
Toxoplasmosis cannot be detected with immunostaining.
Histlogically lymph nodes affected by Toxoplasma have characteristic changes with poorly demarcated reactive germinal centers, clusters of monocytoid B cells, and scattered epithelioid histiocytes.
Congenital toxoplasmosis findings include: chorioretinitis, hydrocephalus, and intracranial arteriosclerosis, with sensorineural deafness, seizures, and intellectual disabilities.
Congenital toxoplasmosis may also impact a child’s hearing
Up to 30% of newborns with congenital toxoplasmosis have some degree of sensorineural hearing loss.
The child’s communication and language skills may also be affected.
Treatment of toxoplasmosis is recommended for people with serious underlying health problems.
Trimethoprim/sulfamethoxazole is the drug of choice to prevent toxoplasmosis, but not for treating active disease.
The medications prescribed for acute toxoplasmosis are the following:
Pyrimethamine — an antimalarial medication
Sulfadiazine — an antibiotic used in combination with pyrimethamine to treat toxoplasmosis
Combination therapy is usually given with folic acid supplements to reduce incidence of thrombocytopaenia.
Clindamycin
Spiramycin used most often for pregnant women to prevent the infection of their children.
Cysts of latent toxoplasmosis are immune to thevabovebtreatments, as the antibiotics do not reach the bradyzoites in sufficient concentration.
The medications prescribed for latent toxoplasmosis are:
Atovaquone
Clindamycin
Acute toxoplasmosis in pregnancy requires amniocentesis to determine whether the fetus has been infected or not., as tachyzoites have approximately a 30% chance of entering the placental tissue, and from there entering and infecting the fetus.
As the gestational age at the time of toxoplasmosis infection increases, the chance of fetal infection also increases.
Spiramycin can help to prevent placental transmission.
If the fetus has been infected, the pregnant woman can be treated with pyrimethamine and sulfadiazine, with folinic acid, after the first trimester(pyrimethamine has an antifolate effect, and lack of folic acid can interfere with fetal brain formation.
Newborns who undergo 12 months of postnatal anti-toxoplasmosis treatment have a low chance of sensorineural hearing loss.
T. gondii infections occur throughout the world, although infection rates differ significantly by country.[25] For women of childbearing age, a survey of 99 studies within 44 countries found the
The areas of highest prevalence of T. gondii are within Latin America (about 50–80%), parts of Eastern and Central Europe (about 20–60%), the Middle East (about 30–50%), parts of Southeast Asia (about 20–60%), and parts of Africa (about 20–55%).
In the United States, data from the National Health and Nutrition Examination Survey (NHANES) found 9.0% of US-born persons 12–49 years of age were seropositive for IgG antibodies against T. gondii.
A trend of decreasing seroprevalence has been observed by numerous studies in the United States.
Toxoplasma gondii is the second leading cause of foodborne-related deaths and the fourth leading cause of foodborne-related hospitalizations in the United States.
Undercooked meat, especially pork, lamb, and wild game meat, and soil contaminated with cat feces on raw fruits and vegetables are the major sources of foodborne transmission.
Free-range organically raised meat could increase the risk of Toxoplasma gondii contamination of meat.
Foodborne transmission can be prevented by adequate cooking of meat, washing of raw fruits and vegetables, prevention of cross contamination in the kitchen, and measures that decrease spread of viable oocysts into the environment.
There are three major types of T. gondii.
There are types I, II, and III.
Type I: is seen in people with AIDS.
Type II: non-virulent mostly in Europe and North America, seen in people with AIDS.
Type III: virulent mainly in animals but seen to a lesser degree in humans.
Seropositivity tests look for the presence of antibodies against T. gondii in blood: seropositivity guarantees one has been exposed to the parasite, it does not necessarily guarantee one is chronically infected.
The Sabin Feldman Dye Test is the gold standard for identifying Toxoplasma infection.
Among livestock, pigs, sheep and goats have the highest rates of chronic T. gondii infection.
Animals kept outdoors or in free-ranging environments are more at risk of infection than animals raised indoors or in commercial confinement operations.
The seroprevalence of T. gondii in domestic cats, worldwide has been estimated to be around 30–40%.
T. gondii infection rates in domestic cats vary with the cats’ diets and lifestyles.
Feral cats are more likely to be infected than domestic cats, and depends on the prevalence of T. gondii-infected prey such as birds and small mammals.
Most infected cats will shed oocysts only once in their lifetimes, for a period of about one to two weeks.
Shedding can release millions of oocysts, each capable of spreading and surviving for months.
An estimated 1% of cats at any given time are actively shedding oocysts.
T. gondii-infected rodents show a number of behavioral changes beyond altered responses to cat odors.
Chronic infection with T. gondii is usually asymptomatic in individuals with normal immune function.
A latent infection may subtly influence a range of human behaviors and tendencies by altering the susceptibility to or intensity of a number of psychiatric or neurological disorders.
Some evidence exists that links T. gondii to schizophrenia: rates of antibodies to T. gondii in people with schizophrenia were 2.7 times higher than in controls: most people with schizophrenia do not have antibodies for toxoplasmosis.
Correlations have also been found between antibody titers to T. gondii and OCD, suicide in people with mood disorders including bipolar disorder.
There is a correlation between T. gondii and many psychological disorders, but the underlying mechanism is unclear.
A study of 236 persons with high levels of Toxoplasmosis antibodies found that T. gondii was not related to increased risk of psychiatric disorder, poor impulse control, personality aberrations or neurocognitive impairment.
Latent Toxoplasmosis infection has been linked to Parkinson’s disease and Alzheimer’s disease.
Individuals with multiple sclerosis show infection rates around 15% lower than the general public.
Latent T. gondii infection is associated with a higher risk of automobile accidents, potentially due to impaired psychomotor performance or enhanced risk-taking personality profiles.
Higher geographic temperatures increase the survival time of T. gondii.
Snowmelt and precipitation can increase the amount of T. gondii oocysts that are transported via river flow, and bird, rodent, and insect population shifts and migration patterns can impact the distribution of T. gondii due to their role as reservoir and vector.
Urbanization and natural environmental degradation are also affect T. gondii transmission and increase risk of infection.

 See Toxoplasma gondii
See Toxoplasma gondii