A specialized primary lymphoid organ of the immune system.
An essential lymphoepithelial organ, and the home for thymocyte (T-cell) development.
Within the thymus, T cells mature.
T cells are critical to the body’s adaptive immune system, allowing it to adapt specifically to foreign invaders.
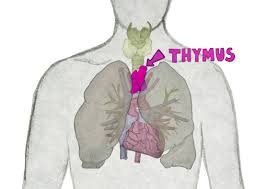
It is composed of two identical lobes and is located anatomically in the anterior superior mediastinum.
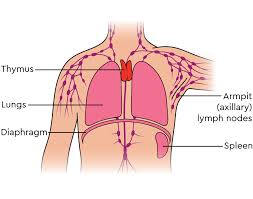
The thymus is located between the inferior neck and the superior thorax.
Histologically the epithelium separates into two compartments, a cortex, and a medulla that correspond to different stages of T cell development.
Early T cell precursors enter the corticomedullary junction and migrate to the cortical periphery for recombination of the variable, dependent, joining loci of the T cell receptor to generate a competent receptor capable of major histocompatibility (MHC) recognition.
The thymus increases in size from birth in response to postnatal antigen stimulation.
At puberty, by the early teens, the thymus begins to atrophy and regress, with adipose tissue mostly replacing the thymic stroma.
However, residual T cell lymphopoiesis continues throughout adult life, providing some immune response.
The thymus is where the T lymphocytes mature and become immunocompetent. Loss/lack of the thymus results in severe immunodeficiency and subsequent high susceptibility to infection.
The thymus consists of epithelium.
T cells mature from thymocytes, proliferate, and undergo a selection process in the thymic cortex before entering the medulla to interact with epithelial cells.
Orchestrates central immune tolerance through selective deletion of effector T cells targeted to self antigens.
Lymphocyte precursors and dendritic cells home to the thymus where cortical epithelial cells promote maturation and lineage commitment.
It is most active during the neonatal and pre-adolescent periods.
Thymus cells can be divided into thymic stromal cells and cells of hematopoietic origin, which are derived from bone marrow resident hematopoietic stem cells.
Developing T cells are referred to as thymocytes and are of hematopoietic origin.
The thymus is critical for normal development of the immune system as infants who undergo thymectomy have reduced T cell counts that do not recover to normal levels, even after years of follow and have impaired immuneresponses to childhood vaccines.
Children with congenital heart defects, who undergo thymectomy have a reduced naïve T cell count that is persistent to adulthood.
Thymus atrophy begins in infancy and accelerates in puberty with a marked decline in new T cell generation.
With decreased central T cell production, the body maintains number of T cells through peripheral clonal proliferation of T cell populations with a limited T-cell receptor base.
Stromal cells include epithelial cells of the thymic cortex and medulla, and dendritic cells.
Central tolerance induction, is a primary goal of the thymus.
While, residual T lymphopoiesis continues throughout adult life, the thymus is largest and most active during the neonatal and pre-adolescent periods.
The thymus begins atrophied and the thymus stroma is mostly replaced by tissue by the early teens.
T cells develop in the thymus from hematopoietic progenitor cells.
Thymus stromal cells promote the selection of a functional and self-tolerant T cell population.
The two lobes differ slightly in size and may be united or separated.
It is in front of the heart and behind the sternum.
The lobe of the thymus can be divided histologically to a central medulla and a peripheral cortex which is surrounded by an outer capsule.
The cortex and medulla have different functions in the development of T cells.
Medullary thymic epithelial cells educate immune cells to recognize self and delete autoreactive T cells before they leave the thymus.
The thymus is about 4-6 cm long, 2.5-5 cm wide, and about 1 cm thick at the time of birth.
It is relatively large in infants which reaches the maximum size of 40 g around the age of three years.
The thymus appears as a pinkish-gray, soft, and lobulated on its surfaces.
In children, the thymus is pinkish-gray, soft, and lobulated on its surfaces.
The thymus is made up of two lobes that meet in upper midline.
It goes from below the thyroid in the neck to as low as the cartilage of the fourth rib.
It lies beneath the sternum and rests on the pericardium.
It is separated from the aortic arch and great vessels by fascia.
The left brachiocephalic vein may even be embedded within the thymus.
In the neck, it lies on the front and sides of the trachea.
It lies behind the sternohyoidei and sternothyreoidei muscles.
The thymus lobes are surrounded by a capsule that extends with blood vessels into the interior.
The thymus consists of a dense outer cortex and an inner less dense medulla.
The lobes are divided into smaller lobules 0.5-2mm diameter.
Thymic tissue sometimes be found scattered on or around the gland.
The cortex is mainly made up of thymocytes, supported by epithelial reticular cells, which is continuous with a similar network in the medulla.
It is in the cortex that the earliest thymocyte development occurs and where T-cell receptor gene rearrangement and positive selection takes place.
Hematopoietic precursors from the bone-marrow, known as thymocytes, mature into T cells mature in the thymus.
The network of reticular cells is coarser in the medulla of the thymus than in the cortex.
The medulla lymphoid cells are relatively fewer in number than in the cortex.
The medulla contains concentric, layered whorls of epithelial cells, called Hassall’s corpuscles.
Hassall’s corpuscles increase in number throughout life, and are the residual of the epithelial tubes, which grow out from the third pharyngeal pouches of the embryo to form the thymus.
Thymocytes reaching the medulla have undergone T-cell receptor gene rearrangement and positive selection, and allow further negative selection to remove auto-reactive T cells from its mature repertoire.
In the medulla maturing thymocytes are exposed to a more complex set of self-antigens than is present in the cortex.
The thymus arterial blood supply is from branches of the internal thoracic, and inferior thyroid arteries, with branches from the superior thyroid artery sometimes seen.
The veins end in the left brachiocephalic vein, internal thoracic vein, and in the inferior thyroid veins.
Lymphatic vessels drain into the brachiocephalic, tracheobronchial and parasternal lymph nodes.
The nerves supplying the thymus arise from the vagus nerve and the cervical sympathetic chain.
Hematopoietic bone-marrow precursors migrate into the thymus, and the resultant interaction between the thymus epithelium and the hematopoietic thymocytes result in the development of thymocytes.
Iodine is also necessary for thymus development and activity.
After birth the thymus reaches its maximum size by the end of the first year of life.
After the first year of life thymic involution occurs with decreased activity, increased peri-vascular space, as the true thymic epithelial space decreases in size.
After puberty it decreases both in size and activity as the thymic peri-vascular space and connective tissue is replaced with fat.
The thymus shrinks by about 3% a year throughout middle age, with a corresponding fall in the thymic production of naïve T cells.
Fat cells initially appear in the walls between lobules, and then slowly spread throughout the cortex and then medulla.
Increased sex hormonal levels are responsible for thymus atrophy.
Conversely, chemical or physical castration of an adult results in the thymus increasing in size and activity.
Mature T cells emigrate from the thymus and make up the peripheral T cells
Peripheral T cells have a significant role in directing many parts of the adaptive immune system.
Loss of the thymus at an early age through genetic mutation resulting in loss of thymus function, as in DiGeorge Syndrome results in severe immunodeficiency and high susceptibility to infection.
Each T cell attacks a specific substance which it identifies with its receptor.
Each T cell attacks a different antigen.
T cells that attack the body’s own proteins are eliminated in the thymus.
T cells undergo “Positive Selection”, as it comes in contact with self-MHC, expressed by thymic epithelial cells.
Those t cels with no interaction die by a lack of stimulatory signal.
The T cell also undergoes “Negative Selection” by interacting with thymic dendritic cells, whereby T cells with a strong interaction with self-MHC and/or self-antigen die by induced apoptosis or are induced to become a regulatory T cell, to avoid autoimmunity.
T cells with intermediate affinity survive.
The thymus is largely degenerated in elderly adults, consisting mostly of fatty tissue.
Thymus involution is related to loss of immune function in the elderly, and susceptibility to infection and to cancer
The T-cell receptor undergoes genetic rearrangement during thymocyte maturation, resulting in each T cell bearing a unique T-cell receptor, specific to a limited set of peptide:MHC combinations.
The ability of T cells to recognize foreign antigens is mediated by the T-cell receptor.
There is requirement of tolerance mechanisms to remove or inactivate those T cells which bear a T-cell receptor with the ability to recognize self-peptides.
Hematopoietic progenitor cells enter the thymus from the blood, and expands by cell division to generate a population of immature thymocytes.
Immature thymocytes each make distinct T-cell receptors by a process of gene rearrangement.
The process of gene rearrangement is subject to errors: failing to make functional T-cell receptors, and making T-cell receptors that are autoreactive.
Immature thymocytes undergo a process of selection, involving selection of T cells that are functional with a positive selection, and elimination of T cells that are autoreactive with a negative selection.
The thymus medulla is the site of T cell maturation.
Thymocytes interact with several cell surface molecules, MHC/HLA, to ensure reactivity and specificity: Positive selection eliminates weakly-binding cells and only takes strongly- or medium-binding cells.
Some autoreactive T cells escape thymic censorship, and are released into the circulation, but additional mechanisms of tolerance active in the periphery exist to silence these cells such as anergy, deletion, and regulatory T cells.
Hormones and cytokines that regulate the maturation of T cells are released from the thymus including thymulin, thymopoietin, and thymosins.
Because the thymus is the organ of T-cell development, any congenital defect in thymic genesis or a defect in thymocyte development can lead to a profound T cell deficiency resulting in a primary immunodeficiency disease.
Defects that affect both the T cell and B cell lymphocyte lineages result in severe combined immunodeficiency (SCID).
Acquired T cell deficiencies can also affect thymocyte development in the thymus.
DiGeorge syndrome is a genetic disorder caused by the deletion of a small section of chromosome 22, and results in a midline congenital defect including thymic aplasia, or congenital deficiency of a thymus.
Patients with DiGeorge syndrome may present with a profound immunodeficiency disease, due to the lack of T cells.
DiGeorge syndrome is the most common congenital cause of thymic aplasia in humans.
Severe combined immunodeficiency syndromes (SCID) are group of rare congenital genetic diseases that result in combined T lymphocyte and B lymphocyte deficiencies.
SCIDs are caused by defective hematopoietic progenitor cells which are the precursors of both B- and T cells.
There is a severe reduction in developing thymocytes in the thymus and consequently thymic atrophy.
Genetic defects that can cause SCID, including IL-7 receptor deficiency, common gamma chain deficiency, and recombination activating gene deficiency.
The HIV virus causes an acquired T-cell immunodeficiency syndrome by specifically killing CD4+ T cells.
One of the primary functions of the thymus is to prevent autoimmunity through the process of central tolerance, immunologic tolerance to self antigens.
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) is an rare genetic autoimmune syndrome.
APECED is caused by mutations in the Autoimmune Regulator (AIRE) gene.
Patients with APECED develop an autoimmune disease that affects multiple endocrine tissues.
Myasthenia gravis is an autoimmune disease caused by antibodies that block acetylcholine receptors, and is often associated with thymic hyperplasia.
Two primary forms of tumours originate in the thymus: Thymomas and Lymphomas.
Thymomas originate from the thymic epithelial cells and are found in about 10-15% of patients with myasthenia gravis.
Thymomas are commonly associated with cough as the tumor presses on the recurrent laryngeal nerve.
Lymphomas or leukemias of thymocyte origin are classified as Precursor T acute lymphoblastic leukemia/lymphoma (T-ALL).
Before 1950, patients with an enlarged thymus, particularly children, were treated with intense radiation resulting in an elevated incidence of thyroid cancer and leukemia.
The most common reason for a thymectomy is to gain access to the heart for surgery to correct congenital heart defects in the neonatal period.
Other indications for thymectomy include: removal of thymomas and the treatment of myasthenia gravis.