 Tauopathy belongs to a class of neurodegenerative diseases involving the aggregation of tau protein into neurofibrillary or gliofibrillary tangles (NFTs) in the human brain.
Tauopathy belongs to a class of neurodegenerative diseases involving the aggregation of tau protein into neurofibrillary or gliofibrillary tangles (NFTs) in the human brain.
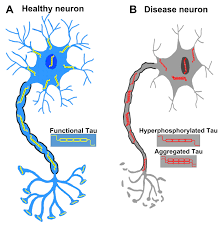
Tangles are formed by hyperphosphorylation of the microtubule protein known as tau, causing the protein to dissociate from microtubules and form insoluble aggregates (helical filaments).
Tau tangles are seen microscopically in stained brain samples.
In living patients tau tangle locations can be imaged with a PET scan using a suitable radio-emissive agent.
Alzheimer’s disease is considered a secondary tauopathy.
AD is also classified as an amyloidosis because of the presence of senile plaques.
With tau hyperphosphorylation, the protein dissociates from the microtubules in axons, and becomes misfolded and aggregates, and eventually forms the neurofibrillary tangles seen in Alzheimer’s patients.
Microtubules also destabilize when tau is dissociated.
The combination of the neurofibrillary tangles and destabilized microtubules result in disruption of axonal transport and neural communication.
Neurofibrillary tangle involvement is defined by Braak stages in AD.
Braak stages I and II are used when NFT involvement is confined mainly to the transentorhinal region of the brain, stages III and IV when there’s also involvement of limbic regions such as the hippocampus, and V and VI when there’s extensive neocortical involvement.
NFTs associated with:
Chronic traumatic encephalopathy (CTE).
Progressive supranuclear palsy (PSP)
Corticobasal degeneration (CBD)
Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17)
Vacuolar tauopathy
Lytico-bodig disease (Parkinson-dementia complex of Guam)
Ganglioglioma and gangliocytoma
Meningioangiomatosis
Postencephalitic parkinsonism
Subacute sclerosing panencephalitis (SSPE)
Lead encephalopathy,
Tuberous sclerosis,
Tauopathies are often overlapped with synucleinopathies, possibly due to interaction between the synuclein and tau proteins.
The tau proteins are a group of six highly soluble protein isoforms produced by alternative splicing from the gene MAPT (microtubule-associated protein tau).
The tau proteins have roles primarily in maintaining the stability of microtubules in axons and are abundant in the neurons of the central nervous system.
Tau proteins interact with tubulin to stabilize microtubules and promote tubulin assembly into microtubules.
Tau has two ways of controlling microtubule stability: isoforms and phosphorylation.
They are also expressed at very low levels in CNS astrocytes and oligodendrocytes.
MAPT Gene location Chromosome 17
The tau proteins are characterized as intrinsically disordered proteins.
Tau proteins are found more often in neurons than in non-neuronal cells.
One of tau’s main functions is to modulate the stability of axonal microtubules.
Although tau is present in dendrites at low levels.
Tau is active primarily in the distal portions of axons, where it provides microtubule stabilization but also flexibility.
Tau has also been found to recruit signaling proteins and to regulate microtubule-mediated axonal transport.
Tau is a negative regulator of mRNA translation in the brain through its binding to ribosomes, which results in impaired ribosomal function, reduction of protein synthesis and altered synaptic function.
Tau interacts with several ribosomal proteins.
Its primary non-cellular function for tau is to negatively regulate long-term memory and to facilitate habituation: possibly explaining the linkage between tauopathies and cognitive impairment.
Other functions of tau include: cellular signalling, neuronal development, neuroprotection and apoptosis.
Atypical roles of tau: involvement in chromosome stability, interaction with the cellular transcriptome, interaction with other cytoskeletal or synaptic proteins, its involvement in myelination or in brain insulin signaling, and its role in the exposure to chronic stress and in depression.
The MAPT gene for encoding tau protein is located on chromosome 17q21, containing 16 exons.
The major tau protein in the human brain is encoded by 11 exons.
In the brain, tau proteins constitute a family of six isoforms with a range of 352–441 amino acids.
The MAPT gene has two haplogroups, H1 and H2.
Haplogroup H2 is common in people with European ancestry.
Haplogroup H1 appears to be associated with increased probability of certain dementias, such as Alzheimer’s disease.
Phosphorylation of tau is regulated by kinases, including PKN.
A serine/threonine kinase, PKN, activated, phosphorylates tau, resulting in disruption of microtubule organization.
Fetal tau is more highly phosphorylated in the embryonic CNS than adult tau.
Phosphorylation in all six isoforms decreases with age due to the activation of phosphatases.
Kinases, and phosphatases play a role in regulating the phosphorylation of tau.
The accumulation of hyperphosphorylated tau in neurons is associated with neurofibrillary degeneration.
Tau aggregation replaces tubulin, which in turn enhances fibrilization of tau.
Tau causes its toxic effects by its accumulation inside cells.
Hyperphosphorylation of the tau protein can result in the assembly of tangles of filaments, which are involved in the pathogenesis of Alzheimer’s disease, frontotemporal dementia and other tauopathies.
All of the six tau isoforms are present in, and are often hyperphosphorylated state in paired helical filaments in the Alzheimer’s disease brain.
In other neurodegenerative diseases, aggregates of certain enriched tau isoforms has been reported.
When misfolded, this soluble protein can form extremely insoluble aggregates that contribute to a number of neurodegenerative diseases.
Tau protein caused by tangles block nerve synapses.
Gender-specific tau gene expression across different regions of the human brain has recently been implicated in gender differences in the manifestations and risk for tauopathies.
It is theorized excessive or abnormal phosphorylation of tau results in the transformation of normal adult tau into paired-helical-filament (PHF) tau and neurofibrillary tangles (NFTs).
The stage of the disease determines NFTs’ phosphorylation.
In Alzheimer Disease at least 19 amino acids are phosphorylated; pre-NFT phosphorylation occurs at serine 119, 202 and 409, while intra-NFT phosphorylation happens at serine 396 and threonine 231.
Through its isoforms and phosphorylation, tau protein interacts with tubulin to stabilize microtubule assembly.
In Alzheimer’s disease all six tau isoforms are present in an often hyperphosphorylated state in paired helical filaments in the brain.
Tau mutations cause microtubule dysfunction and alteration of the expression level of tau isoforms.
Mutations that alter function and isoform expression of tau lead to hyperphosphorylation.
Hyperphosphorylated tau disassembles microtubules and sequesters normal tau, microtubule associated protein tau 1, microtubule associated tau protein 2 and ubiquitin into tangles of paired helical filaments.
The insoluble structures formed damages cytoplasmic functions and interferes with axonal transport, which can lead to cell death.
Hyperphosphorylated forms of tau protein are the main component of paired-helical-filaments (PHF) and neurofibrillary tangles (NFTs) in the brain of AD patients.
An immunoassay blood test for the p-tau-217 form of the protein could diagnose Alzheimer’s up to decades before dementia symptoms were evident.
Repetitive mild traumatic brain injury (TBI) of contact sports and the concussive force of military blasts can lead to chronic traumatic encephalopathy (CTE), a condition characterized by fibrillar tangles of hyperphosphorylated tau.
After severe traumatic brain injury, high levels of tau protein in extracellular fluid in the brain are linked to poor outcomes.
Diseases associated with accumulated tau proteins:
Dementia pugilistica
Alzheimer’s disease
Primary age-related tauopathy
Aging-related tau astrogliopathy
Corticobasal degeneration
Progressive supranuclear palsy
Proteopathy
Pick’s disease
Frontotemporal dementia and parkinsonism linked to chromosome 17
Prion
