
 An intestinal helminthic disease most commonly caused by the parasite S sterocoralis and less commonly by S fuelleborni.
An intestinal helminthic disease most commonly caused by the parasite S sterocoralis and less commonly by S fuelleborni.
S. sterocoralis- roundworm.
A common disease affecting approximately 30-100,000,000 people worldwide:estimate is high is 370 million people worldwide.
In the US, it is most commonly diagnosed in people who have immigrated, or are travelers, returning from high prevalence regions.
S. stercoralis is endemic in the southeastern US.
S stercoralis is considered an intestinal helminth nematode endemic to tropical and subtropical regions that infects humans through contact with soil containing the larvae.
It is a parasitic disease most commonly found in tropical and subtropical countries, where the climate and poor hygiene conditions exist.
S stercoralis Is a nematode that is endemic in large parts of the tropics and subtropics.
Predominantly acquired through contact with soil contaminated with free-living larvae, which penetrate the skin and migrate to the intestine, where they lay eggs.
Infection with S stercoralis typically occurs when the filariform larvae in soil penetrate the skin of individuals who walk barefoot on contaminated soil
Infection begins with inoculation of the skin with filariform larvae reside in the soil.
Larvae migrate through the skin into the bloodstream, which carries them to the lungs.
The larvae migrate through the venous circulation to the lungs, and then ascend the trachea to the oropharynx, where they are swallowed.
After ingestion the larvae lodge in the small intestine and produce eggs that become rhabditiform larvae that are excreted in stool.
The organisms penetrate the alveolar wall and they ascend the bronchi and trachea until they reach the pharynx, where they are swallowed and subsequently reside in the proximal small bowel.
Some larvae, mature into filariform larvae, which penetrate the colonic wall or perianal skin, leading to reinfection of the host, resulting in persistent S stercoralis infection.
Strongyloides completes its lifecycle within the human host.
Larvae mature in the small bowel and colom and then burrow through the bowel wall or perianal skin to restart the lifecycle with in the same host.
The above unique characteristics perpetuates chronic infection, which can last for decades.
Chronic Strongyloidiasis develops when acute Strongyloidiasis is not treated and is perpetuated by autoinfection via the G.I. tract, peritoneum, and lungs, which are directly involved in the lifecycle of S stercoralis.
The infection can therefore last for decades.
Larvae mature into adult worms that inhabit the mucosa of the gut, where they shed eggs that hatch into larvae that eventually excreted in the stool.
Higher prevalence among individuals of low socioeconomic status, in alcoholics, and men.
Like other soil-transmitted helminths the risk of infection is associated with impaired hygeine, making children vulnerable to infection.
Immunocompetent patients are often asymptomatic with chronic Strongyloidiasis, or only have mild abdominal symptoms, minor skin manifestations, and he eosinophilia ranging from 5 to 15%.
Risk factors include: immunosuppressive therapy, malignancy, organ transplants, HIV, alcoholism, tuberculosis, acquired impaired bowel motility, leprosy, and chronic renal failure.
The lifecycle of the parasite begins with a larvae dormant in contaminated soil and enters the host through the skin as one walks barefoot.
The strongyloidiasis worms enter the bloodstream from the bowel wall, and simultaneously allowing entry of bowel bacteria such as Escherichia coli.
This results in sepsis symptoms.
Hyperinfection syndrome, an accelerated auto infection, occurs when patients with chronic Strongyloidiasis develop impaired cell mediated immunity due to steroids, viral infections, chemotherapy, organ transplant, or malnutrition.
Disseminated Strongyloidiasis can develop after hyperinfection, and involves propagation of S steracoralis to organs outside the typical auto infection cycle: skin, brain, heart, liver, gallbladder, pancreas, kidneys, ovaries, and skeletal muscle.
Gastrointestinal manifestations include: epigastric pain, nausea, vomiting, watery diarrhea, malabsorption of fat and vitamin B 12.
Patients with hyperinfection syndrome may experience small bowel obstruction due to large quantities of intestinal, larvae, and G.I. bleeding from intestinal invasion, along with abdominal pain, diarrhea, constipation, nausea and vomiting.
Disseminated infection may be associated with pulmonary symptoms of shortness of breath, wheeze, cough, and hemorrhagic pneumonitis.
Eosinophilia is often absence in hyper infection syndrome, and disseminated Strongyloidiasis.
Diagnosis is based on the microscopic identification of larvae in the stool or duodenal fluid.
The duodenal fluid can be examined using techniques such as the Enterotest string or duodenal aspiration.
In patients with disseminated strongyloidiasis larvae may be detected in sputum.
Walking barefoot is the biggest risk factor.
Larvae enter the alveoli via blood vessels and move upward to the trachea by coughing and are swallowed and settle in the small intestines.
In the small intestines the female parasites mature and begin to lay eggs which release larval forms in to the intestinal lumen and establishes an endogenous infection within the host.
Majority of infected individuals are asymptomatic.
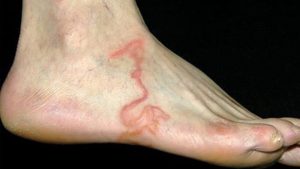
Dermatologic involvement includes urticarial rashes in the buttocks and waist areas as well as larva currens.
Eosinophilia is generally noted.
It can become a chronic process and become completely asymptomatic.
In patients with chronic strongyloidiasis who become immunosuppressed disseminated disease can occur.
Hyperinfection syndrome with an increased worm burden can occur in the context of immunosuppression or HTLV-1 infection.
In the hyper infection syndrome the nematode becomes more invasive, penetrate the bowel mucosa and causes watery but bloody stools and severe abdominal pain.
Dissemination can occur many decades after the initial infection, and may be associated with high dose corticosteroids, organ transplant, and HIV,
Disseminated strongyloidiasis presents with systemic symptoms.
Gastrointestinal symptoms include: abdominal pain and diarrhea.
Respiratory symptoms can occur during pulmonary migration of the filariform larvae.
Strongyloidiasis has a variety of presentations ranging from cutaneous symptoms to gastrointestinal tract symptoms but is asymptomatic in 50% or more of cases.
The only indication of disease may be peripheral eosinophilia.
Causes a number of symptoms in people, principally skin symptoms, abdominal pain, diarrhea and weight loss.
The infection occurs in five forms:
On acquiring the infection, there may be respiratory symptoms.
Chronic infection is associated with mainly digestive symptoms.
On reinfection there may be respiratory, skin and digestive symptoms.
The hyperinfection syndrome causes symptoms in many organ systems, including the central nervous system.
Can result in hyperinfection, dissemination, or death.
Multiple organs can be involved with a chronic infection including the lungs, G.I. tract and skin.
G.I. symptoms range from diarrhea to abdominal pain, bloating and in chronically infected patients a malabsorption syndrome.
Eosinophilia is seen in the majority of patients.
Patients may experience hyperinfection, particularly in patients with immunosuppression with marked increases in parasite numbers and spread to extraintestinal areas.
Hyperinfection as a mortality rate as high as 87%.
Diagnosis can be made by visualization of the larvae on microscopic examination of the stool.
Stool examination requires multiple sample analysis.
Serial examinations are required, as direct stool examination is relatively insensitive, and finding strongyloides in the stool is negative in up to 70% of tests.
Culture techniques are the most sensitive, but are not routinely available.
A duodenal biopsy may be necessary for diagnosis.
An enzyme linked immunosorbent assay test with the testing of IgG antibodies against the parasite is available.
Treatment has been with thiabendazole, but is associated with high relapse rate.
Treatment for uncomplicated disease, the drug of choice is ivermectin.
Ivermectin kills adult worms but not the larvae, and must be repeatedly administered to eradicate the infection.
Other drugs that are effective are: albendazole and thiabendazole.
All patients who are at risk of disseminated strongyloidiasis should be treated.
Mebedazole and albendazole have been used with varying results.
Ivermectin is drug of choice at 200 micrograms per kilogram for two days and is effective in patients of all ages.
The optimal duration of treatment for patients with disseminated infections is not known.
Strongyloides has been known to live in individuals for upwards of 1–2 years after treatment, so that continued treatment may be necessary if symptoms persist.
Treatment should be continued until symptoms resolve themselves.
mortality associated with hyperinfection syndrome and disseminated Strongyloidiasis has been reported in 69 to 85% of cases due to complications such as respiratory failure, peritonitis, meningitis, and bacteremia.
