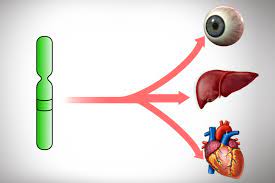
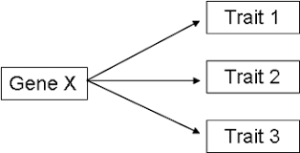
 Pleiotropy refers to a phenomenon in genetics where a single gene has multiple effects or influences on different traits or phenotypes: a single gene can affect multiple seemingly unrelated traits or characteristics.
Pleiotropy refers to a phenomenon in genetics where a single gene has multiple effects or influences on different traits or phenotypes: a single gene can affect multiple seemingly unrelated traits or characteristics.
Pleiotropy describes a phenomenon where a single gene influences multiple traits or has multiple effects on an organism.
In other words, a gene that controls one characteristic can also have an impact on other traits or functions in the body.
Pleiotropy arises from the fact that genes not only control the production of specific proteins or enzymes but can also indirectly affect other biological processes.
These effects can occur due to interconnected pathways and networks within an organism’s physiology, where alterations in one gene can have cascading effects on multiple traits.
Examples: a gene that is responsible for regulating bone development and growth may also influence the development of other tissues or organs, such as the teeth or inner ear; a gene associated with the production of a particular hormone may have effects on various physiological processes throughout the body, including metabolism, development, and behavior.
Pleiotropy concept illustrates the complexity of genetic inheritance and the interconnectedness of biological systems.
Rather than having one gene control one trait, pleiotropy shows that a single gene can have broad-ranging effects on various aspects of an organism’s phenotype.
Pleiotropy can manifest in different ways: susceptibility to certain diseases, influences both height and risk for developing certain types of cancer.
Such diverse effects are often attributed to the gene’s involvement in different metabolic pathways or regulatory mechanisms within the body.
Many genes exhibit pleiotropic effects, affecting various traits across different systems or organs.
Understanding the pleiotropic effects of genes helps elucidate the underlying mechanisms governing complex phenotypes and can provide insights into the interconnectedness of biological processes.
Such a gene that exhibits multiple phenotypic expression is called a pleiotropic gene.
A mutation in a pleiotropic gene may have an effect on several traits simultaneously, due to the gene coding for a product used by a myriad of cells or different targets that have the same signaling function.
Gene pleiotropy occurs when a gene product interacts with multiple other proteins or catalyzes multiple reactions.
Developmental pleiotropy occurs when mutations have multiple effects on the resulting phenotype.
Selectional pleiotropy occurs when the resulting phenotype has many effects on fitness.
An example of pleiotropy is phenylketonuria.
It affects the level of phenylalanine, an amino acid that can be obtained from food, in the human body and causes this amino acid to increase in amount in the body, which can be very dangerous.
The disease is caused by a defect in a single gene on chromosome 12 that codes for enzyme phenylalanine hydroxylase, that affects multiple systems, such as the nervous and integumentary system.
Pleiotropic gene action can limit the rate of multivariate evolution when natural selection, sexual selection or artificial selection on one trait favors one allele, while selection on other traits favors a different allele.
Pleiotropy describes the genetic effect of a single gene on multiple phenotypic traits.
The underlying mechanism is genes that code for a product that is either used by various cells or has a cascade-like signaling function that affects various targets.
Most genetic traits are polygenic in nature: controlled by many genetic variants, each of small effect.
Pleiotropy refers to the influence that a specific genetic variant, e.g., a single nucleotide polymorphism or SNP, has on two or more distinct traits.
A measure of pleiotropy is the fraction of genetic variance that is common between two distinct complex human traits: height vs bone density, breast cancer vs heart attack risk, or diabetes vs hypothyroidism risk.
This has been calculated for hundreds of pairs of traits, which in most cases examined the genomic at least for complex human traits so far examined, pleiotropy is limited in extent.
Pleiotropy can have an effect on the evolutionary rate of genes and allele frequencies.
Studies of pleiotropy suggest have predicted that evolutionary rate of genes is related negatively with pleiotropy: as the number of traits of an organism increases, the evolutionary rates of genes in the organism’s population decrease.
A pleiotropic gene may be both harmful and beneficial to an organism: antagonistic pleiotropy.
Antagonistic pleiotropy: when the trait is beneficial for the organism’s early life, but not its late life: some genes responsible for increased fitness in the younger, fertile organism contribute to decreased fitness later in life, providing and explanation for senescence.
Antagonistic pleiotropy example is the p53 gene, which suppresses cancer but also suppresses stem cells, which replenish worn-out tissue.
Sickle cell anemia is a classic example of the mixed benefit given by the staying power of pleiotropic genes, as the mutation to Hb-S provides the fitness benefit of malaria resistance to heterozygotes, while homozygotes have significantly lowered life expectancy.
Pleiotropy in genes has been linked between certain psychiatric disorders.
Deletion in the 22q11.2 region of chromosome 22 has been associated with schizophrenia and autism.
Schizophrenia and autism are linked to the same gene deletion but manifest very differently from each other.
The resulting phenotype depends on the stage of life at which the individual develops the disorder.
Childhood manifestation of the gene deletion is typically associated with autism, while adolescent and later expression of the gene deletion often manifests in schizophrenia or other psychotic disorders.
Though the disorders are linked by genetics, there is no increased risk found for adult schizophrenia in patients who experienced autism in childhood.
There are genetically linked five psychiatric disorders, including schizophrenia and autism.
There is an associated link with genes autism, schizophrenia and bipolar disorder.
The estimated heritability of schizophrenia is 70% to 90%,
Peiotropy of genes is crucial to the increased risk for certain psychotic disorders and can aid psychiatric diagnosis.
Pleiotropy occurs is the disease phenylketonuria (PKU).
PKU causes mental retardation and reduced hair and skin pigmentation, and can be caused by any of a large number of mutations in the single gene on chromosome 12 that codes for the enzyme phenylalanine hydroxylase, which converts the amino acid phenylalanine to tyrosine.
The conversion of the amino acid phenylalanine to tyrosine is reduced or ceases entirely with the unconverted phenylalanine building up in the bloodstream to levels that are toxic to the developing nervous system of newborn and infant children.
In classic PKU, which is common in infants, the baby seems normal at first but actually incurs permanent intellectual disability.
It can cause symptoms such as mental retardation, abnormal gait and posture, and delayed growth.
Failure to convert normal levels of phenylalanine to tyrosine can lead to fair hair and skin because tyrosine is used by the body to make melanin
PKU is found at a rate of nearly 1 in 10,000 births.
Due to newborn screening, we are able to detect PKU in a baby sooner. allowing early treatment preventing the effects of PKU.
PKU is caused by a mutation in the PAH gene that instructs the body on how to make phenylalanine hydroxylase that converts phenylalanine, taken in through diet, into other things that the body can use.
Phenylalanine is ingested through food, so a diet should decrease types of foods that have high amounts of phenylalanine: breast milk, eggs, chicken, beef, pork, fish, nuts.
Sickle cell anemia is a genetic disease that causes deformed red blood cells with a rigid, crescent shape instead of the normal flexible, round shape.
Sickle cell anemia is caused by a change in one nucleotide, a point mutationmin the HBB gene.
The HBB gene encodes information to make the beta-globin subunit of hemoglobin.
Sickle cell anemia occurs when the HBB gene mutation causes both beta-globin subunits of hemoglobin to change into hemoglobin S (HbS).
Sickle cell anemia is a pleiotropic disease because the expression of a single mutated HBB gene produces numerous consequences throughout the body.
The mutated hemoglobin forms polymers and clumps together causing the deoxygenated sickle red blood cells to assume the disfigured sickle shape.
Red blood cells are inflexible and cannot easily flow through blood vessels, increasing the risk of blood clots and possibly depriving vital organs of oxygen.
Some complications associated with sickle cell anemia include pain, damaged organs, strokes, high blood pressure, and loss of vision.
Sickle red blood cells also have a shortened lifespan and die prematurely.
Marfan syndrome (MFS) is an autosomal dominant disorder which affects 1 in 5–10,000 people.
MFS arises from a mutation in the FBN1 gene, which encodes for the glycoprotein fibrillin-1, a major constituent of extracellular microfibrils which form connective tissues.
Over 1,000 different mutations in FBN1 have been found to result in abnormal function of fibrillin, which consequently relates to connective tissues elongating progressively and weakening.
Because these fibers are found in tissues throughout the body, mutations in this gene can have a widespread effect on certain systems, including the skeletal, cardiovascular, and nervous system, as well as the eyes and lungs.
Without medical intervention, prognosis of Marfan syndrome can range from moderate to life-threatening,
90% of known causes of death in diagnosed patients relating to cardiovascular complications and congestive cardiac failure.
Other characteristics of MFS include an increased arm span and decreased upper to lower body ratio.
