 Utilizes the peritoneum as the artificial kidney.
Utilizes the peritoneum as the artificial kidney.
An estimated 3.8 million people worldwide currently rely on some form of dialysis for the treatment of end-stage kidney disease (ESKD).
Peritoneal dialysis accounts for approximately 11% of patients undergoing dialysis overall.
In developed countries peritoneal dialysis is less expensive to deliver than hemodialysis.
The use of peritoneal dialysis for several advantages over hemodialysis: better preservation of residual kidney function, fewer volume shifts, and more flexibility.
Successful peritoneal dialysis requires that patients be motivated and cognitively able and must have a preserved peritoneal anatomy.
The peritoneum histologically consists of a single layer of mesothelial cells resting on a submesothelial interstitial tissue, a gel like matrix containing fibroblasts, adipocytes, collagen fibers, nerves, lymphatic vessels, and capillaries.
The endothelium of the peritoneum‘s capillaries functions is the filter that regulates peritoneal transport.
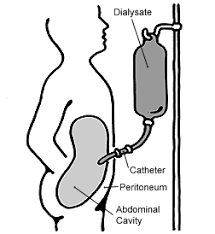
Peritoneal dialysis involves diffusion and osmosis through a peritoneal membrane, measuring 1 m², that is highly vascularized with the flow rate of 100 to 150 mL per minute and has a total available capillary surface area of approximately 2 m².
The peritoneum therefore provides a suitable membrane for the performance of dialysis.
The peritoneum approximates body surface area in size.
Clean dialysate is placed in the patient’s abdominal cavity via a surgically placed catheter, it contans favorable fluid and electrolyte concentrations and remains in the abdomen for a number of hours, and is then drained removing metabolic wastes and other toxins that have diffused out of the blood into the fluid.
Water moves from the blood into the dialysate via osmosis and the osmotic pressure gradient is generated by a high concentration of glucose in the dialysate, drawing water into the abdominal compartment.
In individuals with less peritoneal vascularity solutes diffuse slowly in both directions:waste products accumulate in the dialysate slowly in the glucose gradient favoring ultrafiltration dissipates slowly.
Onversely, patients with greater peritoneal vascularity the solute diffuse more rapidly, also in both directions and the glucose gradient favoring ultrafiltration dissipates more rapidly.
Glucose has been used as the prototypical crystalloid osmotic agent to drive water removal in peritoneal dialysis.
Glucose is a small osmotic agent with a molecular weight of 180 g per mole that is prone to crystallization but easily diffuses across semi permeable membranes, hence it is known as a crystalloid substance.
In contrast choloid and non-crystalloid substances are retained by membranes because they are large.
Solutes diffuse from the blood in the peritoneal capillaries into the dialysate, effecting in an exchange analogous to that of extracorporeal hemodialysis.
Similarly, imposition of a transmembrane pressure gradient creates the driving force for ultrafiltration of fluid from the capillaries into the dialysate.
Unlike hemodialysis, in which the pressure is applied is hydrostatic, peritoneal dialysis involves osmotic pressure created by the intraperitoneal installation of hypertonic dialysate, usually as glucose in the form of 1.5, 2.5, or 4.25% dextrose.
The higher the concentration of glucose exerts a higher osmotic pressure and effects greater degrees of ultra filtration.
Peritoneal dialysis uses the peritoneum as a biological membrane for solute and fluid removal.
The process exchanges solute and volume between the extracellular fluid in the peritoneal dialysis solution across the peritoneal membrane.
Sodium transfer is across the peritoneal capillaries bidirectionally.
Solutes, such as urea, creatine, and potassium diffuse from the bloodstream into the dialysate, whereas glucose diffuses from the dialysate into the peritoneal capillaries.
The diffusion of glucose out of the dialysate into the peritoneal capillaries results in dissipation of the osmotic gradient and progressive slowing in the rate of ultrafiltration.
The transfer rate of solute across the peritoneum depends on the concentration gradient and the degree of peritoneal vascularity, which varies from person to person.
To prevent pericatheter leakage, peritoneal dialysis is started after a few weeks after tube placement.
An electrolyte in glucose containing solution fills the peritoneal cavity and is allowed to dwell there.
Hydrothorax may appear in the right chest cavity via a diaphragmatic defect.
Risk of infection is extremely low and the international Society of Peritonal Dialysis recommends the monitoring of peritoneal dialysis programs to achieve rates of infection lower them one case per 18 patient months.
It is associated with treatment specific complications including infection-related and mechanical.
Infections and cardiovascular complications are the most frequent causes of hospitalizations in peritoneal dialysis patients.
Associated with 2 hospitalizations/year.
Fluid exchanges can be done several times a day via continuous ambulatory peritoneal dialysis.
Efficacy can be limited by recurrent peritonitis and loss of peritoneal clearance or residual renal function.
Infection can occur with a breach in aseptic technique that introduces infectious organisms into the peritoneal dialysis catheter, the catheter exit site or tunnel infection with skin organisms that might migrate into the peritoneal cavity, translocation of organisms from the G.I. or GU tract, or, in less than 1% of cases hematogenous spread to the peritoneal cavity.
When bacterial organisms enter the peritoneal cavity, polymorphonuclear leukocytes induce an inflammatory reaction that causes abdominal pain and a cloudy dialysate.
Residual renal function is better preserved with peritoneal dialysis than with hemodialysis.
30-45% of patients will develop acquired cystic disease and of those 5-30% may develop renal cancer.
7% of patients on dialysis are on peritoneal dialysis.
Peritoneal dialysis is cheaper than hemodialysis by approximately $20,000 per patient per year.
Patients to start on PD are usually younger, healthier, more likely to be employed than are patients starting hemodialysis.
It is suggested than older patients with diabetes and congestive heart failure do not do as well on PD.
Over the last 8 years there has been experiencing reduction in mortality rate for new patients starting PD in the US.
In the most recent studies patients starting on PD compared to patients hemodialysis have similar outcomes (Mehrotra R et al).
Peritonitis in patients with peritoneal dialysis manifests with abdominal pain, fever, nausea, vomiting, diarrhea, tenderness, and rebound tenderness on clinical exam.
Peritoneal dialysis associated peritonitis is diagnosed with the clinical features noted above and or with a cloudy peritoneal fluid, a fluid with white blood cell count greater than equal to 100 cells per microliter with greater than or 50% of neutrophils in the dialysate.
Peritoneal dialysis associated peritonitis occurs in approximately 30 to 40% of patients during the treatment course and develops when infectious organism gains access to the peritoneal cavity.
Peritoneal dialysis programs should strive to achieve infection rates lower than one case per 18 patient months.
Infections and cardiovascular complications are the most frequent causes of hospitalization in patients on peritoneal dialysis.
Corraborating tests for peritonitis on PD include an elevated white blood cell evaluation on peripheral blood and peritoneal fluid analysis.
Cloudy peritoneal fluid maybe seen with active peritonitis or chemical peritonitis, malignant processes or chylous fluid.
With suspicion of the presence of peritoneal infection antibiotics should be started empirically.
Antibiotics can be delivered intraperitoneally or intravenously and should cover both gram-positive and gram-negative organisms.
Peritoneal fluid within elevated white count of greater than 100 cells per high-powered field is consistent with the diagnosis of peritonitis.
Empirical antibiotic coverage should include gram-positive andvgram-negative coverage: agents should either be first-generation cephalosporins or vancomycin and third-generation cephalosporins, quinolone or gentamicin..
