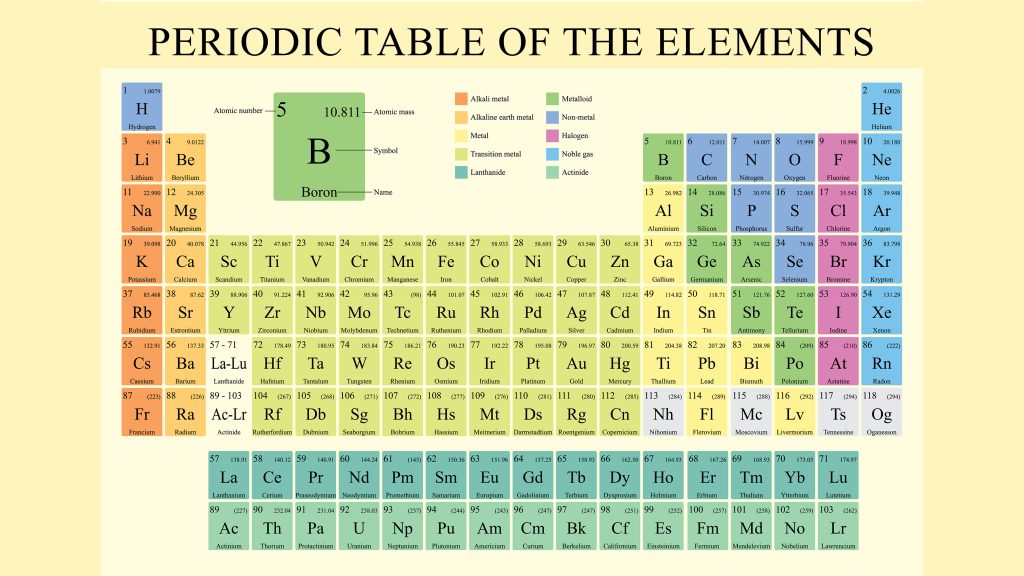
The Periodic Table of Elements
Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon Caesium Barium Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury (element) Thallium Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Copernicium Nihonium Flerovium Moscovium Livermorium Tennessine Oganesson
The periodic table, also known as the periodic table of the elements, is a rows and columns arrangement of the chemical elements.
The periodic table is a tabular arrangement of the chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties.
It is a visual representation of the known elements, showing their names, symbols, atomic numbers, and atomic masses.
The table is divided into rows called periods and columns called groups.
The periodic table helps scientists and chemists understand the relationships and patterns between different elements.
It provides information about the elements’ properties, such as their reactivity, atomic structure, and the arrangement of their electrons.
By studying the periodic table, researchers can predict the behavior of elements, their compounds, and their reactions with other substances.
The periodic table is a table of elements arranged in such a way that the elements with similar chemical properties appear in vertical columns called groups or families, and the elements with same number of electron shells appear in horizontal rows called periods.
The table is arranged in order of increasing atomic number, which is the number of protons in the nucleus of an atom of the element.
Each element is represented by a symbol, which is usually an abbreviation of its name.
The symbols are arranged in the table so that elements in the same group or period tend to have similar electronic configurations and exhibit similar chemical properties.
The periodic table is an important tool in chemistry, as it helps to predict the properties of elements and their compounds, and it provides a framework for understanding chemical reactions and patterns in the behavior of elements.
It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry.
It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers.
The table is divided into four roughly rectangular areas called blocks.
The rows of the table are called periods, and the columns are called groups.
Elements from the same group of the periodic table show similar chemical characteristics.
Trends run through the periodic table.
The nonmetallic character of keeping their own electrons increases from left to right across a period, and from down to up across a group, and metallic character, that is, surrendering electrons to other atoms increases in the opposite direction.
The periodic table exclusively lists electrically neutral atoms that have an equal number of positively charged protons and negatively charged electrons and puts isotopes, atoms with the same number of protons but different numbers of neutrons, at the same place.
Other atoms, like nuclides and isotopes, are graphically collected in other tables.
The periodic table and law are now a central and indispensable part of modern chemistry.
Today, all the first 118 elements are known, completing the first seven rows of the table, but chemical characterization is still needed for the heaviest elements to confirm that their properties match their positions.
The periodic table is a 2-dimensional structured table.
The elements are placed in table cells, in reading order of ascending atomic number.
The table is divided into four blocks, reflecting the filling of electrons into types of subshell.
The period table columns are called groups, and the rows are called periods.
New periods begin when a new electron shell starts to fill: elements in the same group have the same number of electrons that can be used for chemistry so that similar physical and chemical properties recur at regular intervals.
The smallest constituents of all normal matter are known as atoms.
Atoms are extremely small, being about one ten-billionth of a meter across, and
their internal structure is governed by quantum mechanics.
Atoms consist of a small positively charged nucleus, made of positively charged protons and uncharged neutrons, surrounded by a cloud of negatively charged electrons; the charges cancel out, so atoms are neutral.
Electrons participate in chemical reactions, but the nucleus does not.
When atoms participate in chemical reactions, they either gain or lose electrons to form positively- or negatively-charged ions; or share electrons with each other.
Atoms can be subdivided into different types based on the number of protons (and thus also electrons) they have.
This is called the atomic number, often symbolized Z.
Each distinct atomic number therefore corresponds to a class of atom: these classes are called the chemical elements.
The chemical elements are what the periodic table classifies and organizes.
Hydrogen is the element with atomic number 1; helium, atomic number 2; lithium, atomic number 3; and so on.
Each of these names can be further abbreviated by a one- or two-letter chemical symbol; those for hydrogen, helium, and lithium are respectively H, He, and Li.
Neutrons do not affect the atom’s chemical identity, but do affect its weight.
Atoms with the same number of protons but different numbers of neutrons are called isotopes of the same chemical element.
Naturally occurring elements usually occur as mixes of different isotopes.
Each isotope occurring elements have well-defined atomic weights, defined as the average mass of a naturally occurring atom of that element.
118 elements are known, the first 94 of which are known to occur naturally on Earth at present.
Of the 94 natural elements, eighty have a stable isotope.
Bismuth has an almost-stable isotope with a half-life over a billion times the age of the universe.
Two more, thorium and uranium, have isotopes undergoing radioactive decay with a half-life comparable to the age of the Earth.
The stable elements plus bismuth, thorium, and uranium make up the 83 primordial elements that survived from the Earth’s formation.
The remaining eleven natural elements decay quickly enough that their continued trace occurrence rests primarily on being constantly regenerated as intermediate products of the decay of thorium and uranium.
All 24 known artificial elements are radioactive.
The periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number.
Elements are placed in the periodic table by their electron configurations, which exhibit periodic recurrences that explain the trends of properties across the periodic table.
An electron can be thought of as inhabiting an atomic orbi.
The possible states an electron can take in various energy levels known as shells, divided into individual subshells, which each contain one or more orbitals.
Each orbital can contain up to two electrons: they are distinguished by a quantity known as spin: “up” or “down”.
A cold atom, in its ground state has electrons arranged in such a way that the total energy they have is minimized by occupying the lowest-energy orbitals available.
Only the outermost electrons, the valence electrons, have enough energy to break free of the nucleus and participate in chemical reactions with other atoms.
The first eighteen elements can thus be arranged as the start of a periodic table.
Elements in the same column have the same number of valence electrons and have analogous valence electron, these columns are called groups.
There are eight columns in this periodic table fragment, corresponding to at most eight outer-shell electrons.
The coloring illustrates the blocks: the elements in the s-block, colored red are filling s-orbitals, while those in the p-block colored yellow are filling p-orbitals.
Under an international naming convention, the groups are numbered numerically from 1 to 18 from the leftmost column (the alkali metals) to the rightmost column, the noble gases.
Elements in the same group share the same valence configurations, and usually exhibit similar chemical behavior.
Electronegativity is an important property of elements.
The metallicity of an element can be predicted from electronic properties.
The elements in the periodic table are organized based on their atomic structure and properties.
Elements are arranged in order of increasing atomic number, which is the number of protons in the nucleus of an atom.
The atomic number determines an element’s identity.
Elements in the periodic table are organized into columns called groups or families.
Elements within the same group have similar chemical properties due to having the same number of electrons in their outermost energy level or valence electrons: There are 18 groups in the periodic table.
Elements are also arranged in rows called periods.
Elements in the same period have the same number of electron shells.
As you move from left to right acthe atomic number increases, and chemical properties change in a predictable manner.