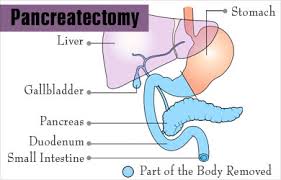
 Pancreatectomy is a term for surgical removal of all or part of the pancreas.
Pancreatectomy is a term for surgical removal of all or part of the pancreas.
There is a multitude of surgical techniques for both benign and malignant processes of the pancreas, including different types of surgical excision.
Pancreatic surgery is technically challenging and requiring significant experience and excellent clinical judgment from the surgeon.
The pancreas is an oblong glandular abdominal organ that has both endocrine and exocrine functions.
The organ lies in the retroperitoneum while crossing the body of L1 and L2 along the posterior aspect of the abdominal wall.
The pancreas lies in a transverse plane between the C loop of the duodenum while the tail of the pancreas touches the hilum of the spleen.
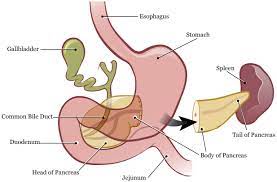
It is divided into five regions, which consist of a head, uncinate process, neck, body, and tail.
The head of the pancreas is the widest part of the organ.
The uncinate lies underneath the body of the pancreas, coming off the posteromedial area of the pancreatic head and curving just posterior to the superior mesenteric artery and vein.
The head of the pancreas lies just to the right of the superior mesenteric vessels, and it is attached to the second and third portions of the duodenum. The uncinate process lies adjacent to the third and fourth portions of the duodenum.
The uncinate is absent in some people, can vary in size and thickness, and has also been noted to encircle the superior mesenteric vessels in others.
The neck begins at the passage of the superior mesenteric vessels and spleno-portal vein confluence posterior to the gland.
The neck is the thinnest portion of the pancreas and can be crushed in blunt trauma by being compressed against the second lumbar vertebrae, which sit just posterior to the neck.
The body of the pancreas is covered anteriorly by the omental bursa, which separates the stomach from the pancreas.
The body lies just to the left of the aorta.
The tail of the pancreas is the most mobile aspect of the gland.
The tail usually rests in the hilum of the spleen or just below in the majority of people.
The majority of pancreatic enzymes are drained through the main pancreatic duct, which then drains out the common channel, which is formed after the meeting of the common bile duct and main pancreatic duct.
The common channel length is variable in many patients.
The superior and anterior portions of the pancreatic head are drained by the accessory duct of Santorini, with the remaining portions of the pancreas being drained by the main pancreatic duct of Wirsung.
After the main pancreatic duct and common bile duct join, they enter into the 2nd portion of the duodenum and drain through the ampulla of Vater.
The ampulla is surrounded by a muscular ring called the sphincter of Oddi, which controls the flow of bile and pancreatic fluid into the duodenum.
The sphincter of Oddi is under neural and hormonal control.
The sphincter of Oddi, is very sensitive to cholecystokinin, which is produced in the duodenum and causes relaxation of the sphincter.
There is often a second duct that drains into the duodenum, called to the minor papilla.
The minor papilla is actually located superior to the Ampulla of Vater in the wall of the duodenum.
The vascular supply of the pancreas is very complex and is derived from both the celiac artery and the superior mesenteric artery.
The common hepatic artery and splenic artery both arise from the celiac trunk.
The common hepatic artery gives rise to the gastroduodenal artery (GDA); the GDA then gives rise to the right
gastroepiploic artery after which it continues inferiorly becoming the anterior and posterior superior pancreaticoduodenal arteries.
The superior mesenteric artery supplies both the inferior anterior and posterior pancreaticoduodenal arteries.
These two superior and inferior pancreaticoduodenal arteries supply the pancreatic head, 2nd, and 3rd portions of the duodenum.
The excellent blood supply to this area allows duodenal sparing pancreatic surgery to be performed.
The remaining portions of the pancreas are supplied by the splenic artery and superior mesenteric artery.
The splenic artery runs along the superior aspect of the pancreas and gives off many branches.
Arteries arise from the splenic artery, which run perpendicular across the pancreatic body and tail.
The three arteries of the splenic artery help form collateralization between the splenic artery and the inferior pancreaticoduodenal arteries.
When planning pancreatectomy high-quality CT imaging must, view the pathological disease process (i.e., cancer or lesion) but to required visualization of the vessels during surgical planning.
Determining resectability involves the
vascular determinations:
abutment of the superior mesenteric artery, celiac artery, or hepatic artery, all less than 180 degrees, or have very small areas that are occluded along the portal vein, the superior mesenteric vein may possibly require reconstruction.
Resectability is based on the probability of obtaining a margin negative resection (RO) by assessing circumferential degrees of contact, between the tumor and the arterial and venous structures (superior, mesenteric artery, celiac access, and common hepatic artery and venous portal or superior mesenteric vein structures).
Resectable pancreatic cancer is defined as a tumor vein contact of less or equal to 180° without vein contour irregularity, and no arterial contact.
Borderline resectable disease is defined as a tumor-vein contact of 180° or less with vein contour irregularity, a tumor artery contact of 180° or less, tumor – superior mesenteric, vein, or a portal vein contact of greater than 180°, but the site of involvement allows for safe and complete resection and vein reconstruction.
Unresectable/locally advanced, pancreatic cancer, refers to a tumor surrounding the vessel with greater than 180°, which surgery will have a low probability of obtaining an RO margin.
Tumors that circumferentially encase the celiac artery, hepatic artery, or the superior mesenteric artery greater than 180 degrees or have complete occlusion of the superior mesenteric vein or portal vein with no possibility of reconstruction are considered locally advanced and surgically unresectable.
CT imaging information about anatomical variants that may be present: A replaced right hepatic artery is the most common arterial variant, with the artery arising from the superior mesenteric artery (15% of the population).
A replaced left hepatic artery occurs in less than 10% of the population.
The venous drainage of the pancreas usually mirrors the arterial supply.
The veins are usually located anteriorly to the arteries, while the pancreatic ducts are anterior to both arteries and veins.
The venous drainage goes into the superior mesenteric vein, inferior mesenteric vein, splenic vein, or portal vein.
Like the arterial system to the body and tail, three major veins drain these areas.
The inferior pancreatic, caudal, and great pancreatic veins all drain directly into the splenic vein.
These veins are of great importance when a spleen preserving distal pancreatectomy is performed as all these branches must be divided and ligated.
Lymphatic drainage of the pancreas aligns with the vessels supplying the gland.
Metastatic disease from the pancreas is present in up to 70% of patients in their lymph nodes.
The pancreas’ lymphatic system consists of five main groups referred to as the anterior, posterior, superior, inferior, and splenic lymph nodes.
The superior half of the pancreatic head drains into the superior lymph nodes while the inferior portion of the pancreatic head drains into the inferior lymph nodes.
The pancreatic head also drains to a portion of nodes located at the right side of the celiac and superior mesenteric arteries.
The pancreatic body is divided into a superior and inferior portion for lymph drainage; the superior aspect drains into the superior nodes while the inferior portion drains into inferior pancreatic, superior mesenteric, and also para-aortic lymph nodes.
The tail of the pancreas drains directly into the splenic lymph nodes.
Both sympathetic and parasympathetic nerves innervate the pancreas.
The nerves follow the pathway of the vessels that supply the pancreas.
The vagus nerve is the parasympathetic innervation which comes from the celiac plexus.
The vagus nerve is mainly a sensory nerve in its function.
The sympathetic innervation begins in the thoracic spinal cord then runs through the greater and lesser splanchnic nerves.
Pancreatic pain is mainly transmitted through the celiac plexus.
Celiac nerve blocks can be used as a treatment for chronic pain in the setting of locally advanced cancer.
The pancreas performs both endocrine and exocrine functions.
The endocrine portion only contributes 1% to 2% of the gland’s function, while the majority of the pancreas is used for exocrine function.
The endocrine portion is responsible for glucose homeostasis.
The exocrine function is involved with the secretion of enzymes involved in digestion.
The exocrine pancreas has a functional unit called an acinus.
The acinus has zymogen granules that subsequently drain into the main pancreatic duct.
The acinar cells secrete either amylases, lipases, or proteases.
The secretion of these enzymes is due to the parasympathetic system, secretin, and CCK stimulation.
Approximately 50% of the pancreatic acinar cells have to be damaged before there is an effect on their digestion function.
The acinar cell can secrete each of these enzymes, which ultimately appears as an alkaline fluid that is colorless and has no odor.
The pancreas drains between 1 to 2 liters of exocrine fluid per day, which has an alkalotic pH due to the high bicarbonate concentration in the fluid.
As pancreatic stimulation increases, so do the rate of bicarbonate, but chloride concentration decreases.
The islets of Langerhans perform the pancreatic endocrine function.
There are nearly one million islets in the normal functioning adult pancreas.
These islets consist of 5 cell types- alpha cells that secrete glucagon, beta cells that secrete insulin, delta cells that secrete somatostatin, epsilon cells that secrete ghrelin, and F or PP cells that secrete pancreatic polypeptide.
The hormones are released in a balanced mixture into the portal vein in reaction to changes in the plasma levels.
The majority of the endocrine cells are located in the islet cell periphery, while the beta cells are located in the center and comprise 70% of the islet mass.
Alpha cells are predominantly seen in the islet cells in the superior aspect of the pancreatic head, body, and tail; it accounts for 10% of the islet mass.
Delta cells are like beta cells, which are present in all islets throughout the pancreas.
Delta cells make up only 5% of the islet cell mass.
PP cells are mostly seen in the islets in the pancreas’ head and account for 15% of the islet mass.
Surgical planning must take into account the physiological function of the pancreas that will remain.
Some patients may require exogenous administration of pancreatic digestive enzymes after surgery or insulin administration with frequent glucose monitoring.
Roughly 20 % of the pancreas volume is needed in the remnant to avoid endocrine or exocrine insufficiency.
Indications regarding laparoscopic vs open pancreatectomy are relatively the same.
Total Pancreatectomy:
Malignant tumors of the pancreatic head with involvement in the left pancreas
Unable to obtain tumor-free R0 resections at the pancreatic margin
Unable to perform pancreatic anastomosis after pancreaticoduodenectomy
Recurrent pancreatic cancer in the remnant pancreas
Removal of the remaining pancreas after Whipple complication of bleeding or leak
multifocal intraductal papillary mucinous neoplasm (IPMN) in all parts of the pancreas
multifocal neuroendocrine tumors of the pancreas with a history of multiple endocrine neoplasias
Hereditary pancreatic cancer or Family history
Intractable pain due to chronic pancreatitis or multiple bouts of recurrent acute pancreatitis
Distal Pancreatectomy indicated for
benign or malignant tumors involving the body or tail of the pancreas located to the left side of the superior mesenteric vein.
Chronic pancreatitis confined to the body or tail
Pseudocyst involving the tail of the pancreas
Trauma to the distal pancreas
Ductal disruption or stricture +/- pancreatic fistula in body or tail
Central Pancreatectomy
Benign or borderline lesions located in the neck or proximal body of the pancreas
Enucleation of the lesion unable to be performed at the neck/proximal body
Trauma-related injury to the neck/proximal body
Contraindications
Medical comorbidities that prohibit operative intervention:
Poor functional status
Bleeding coagulopathy
Poor Laparoscopic Technique
Severe obesity
Previous major abdominal surgeries
Advanced malignancy Involving vascular structures
Unable to tolerate pneumoperitoneum
Total Pancreatectomy
Lesions that are amenable for removal with sparing of pancreatic tissue by another resection type
Patients undergoing cancer resections should have preoperative Ca19-9 check for three reasons:
If elevated confirming concern for malignancy
If exceedingly high raise suspicion for disseminated disease
A starting point to be utilized as a reference for post-operative monitoring.
CT imaging can provide the precise location of the lesion, size, and, most importantly, any involvement of any surrounding structures.
CT imaging can really help determine the resectability of the tumor.
A pancreatic CT protocol involves triphasic cross-sectional imaging with arterial, late arterial, and venous phases.
It allows identification of the majority of pancreatic cancers in the delayed arterial phase/venous phase, as many are hypovascular in nature.
CT imaging has a reported sensitivity ranging from 76-92% for diagnosing pancreatic cancer and surgical resectability up to 90%.
The ultrasound can be used to look for vascular invasion and resectability of the mass.
Endoscopic is best used to obtain a tissue biopsy, and helps diagnose nodal involvement and metastatic diseases, such as with large lymph nodes in the celiac or mediastinal positions.
MRI is an excellent modality for determining pancreaticobiliary anatomy and pathology.
MRCP can identify pancreatic duct abnormalities, such as strictures. It is better overall at identifying the biliary and pancreatic ductal systems.
When CT and PET scans are combined, this can increase the overall accuracy while increasing the sensitivity and specificity, especially for lesions that were missed on CT imaging.
Many surgeons are now using either robotic or laparoscopic techniques to perform central, distal, or enucleation resections on the pancreas.
Laparoscopic techniques offer the patient less postoperative pain, shorter hospital stay, less intraoperative blood loss, quicker return to daily activities, less postoperative ileus, and overall fewer surgical complications.
If the patient is scheduled for a pancreatic procedure due to a malignant lesion, the patient usually undergoes a diagnostic laparoscopy first, making sure to explore the abdomen making sure there is no unseen metastatic disease noted to the peritoneum, liver, or surrounding structures.
Peritoneal washings can be taken at this time.
Once no metastatic disease has been established, the laparoscopic technique can continue, or the team can convert to open for the resection or proceed in a minimally invasive fashion if this is their usual surgical technique.
Exploring the abdomen should again be performed to definitely rule out any metastatic disease findings by inspecting the liver, small bowel, transverse colon, mesentery, diaphragm, and peritoneum.
A central pancreatectomy allows the surgeon to conserve pancreatic parenchyma if the given lesion is operable with this technique.
Central pancreatectomy allows much more conservation of the pancreas with postoperative long-term diabetes outcomes, only being 11 to 12% versus 20 to 50% of distal pancreatectomy patients.
The exocrine function is also maintained at a much higher rate with central pancreatectomy, with only 10% of patients requiring supplementation versus 27% of distal pancreatectomy patients.
Central pancreatectomy also preserves the entire biliary anatomy and avoids splenectomy.
Central pancreatectomy is not indicated for pancreatic cancer as margins that remain may harbor malignancy, and lymph node harvest is not as sufficient.
Surgical intervention for central pancreatectomy is only reserved for benign or potential borderline lesions and is thus probably the least likely utilized pancreatic resection surgery.
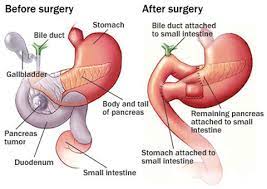
The distal remnant pancreas can then be drained either into the stomach or into the small bowel.
If a pancreaticogastrostomy is the choice for drainage, then the pancreatic body must be mobilized off the splenic vessels for better mobilization to the stomach.
The distal jejunum continuity can be restored with a jejunojejunostomy anastomosis.
The pancreatic duct to jejunum anastomosis can be performed with an end to side anastomosis.
Distal Pancreatectomy
A pancreatic transection can be performed, ranging from the neck to the distal tail.
Distal Pancreatectomy with islet cell autotransplantation has become much more commonly performed after total pancreatectomy for chronic pancreatitis.
Total pancreatectomy removes all endocrine and exocrine function from the patient.
Autotransplantation allows the patient to have an endocrine function even after the removal of the pancreas.
Contraindications to performing total pancreatectomy with islet autotransplantation: if the patient has an active substance abuse, pancreatic cancer, or poorly controlled psychiatric illnesses.
With total pancreatectomy and islet autotransplantation, the transplantation can occur during the same operative intervention.
The goal of total pancreatectomy with islet autotransplantation is to relieve the patient of chronic pain and to reserve their endocrine function to avoid chronic insulin use.
Pancreatic injury due to blunt or penetrating trauma, may require surgical intervention depending on ductal injury and hemorrhage control in the operating room.
CT imaging is of great importance for diagnosing pancreatic injuries.
With any trauma-related injury to the pancrea, the goal is to control hemorrhage and avoid further gastrointestinal spillage into the abdomen.
The surgeon must have complete exposure of the entire gland.
The majority of pancreatic injuries involve minor contusions, hematomas near or on the gland, and at times lacerations to the capsule.
The main goal of managing the pancreatic injury is to identify if there is a pancreatic duct injury.
Postoperative pancreatic complications can gravely affect the patient if not diagnosed and managed in a quick manner.
Postoperative fistulas are one of the most common complications occurring after pancreatectomy.
Some reports have a fistula rate occurring as high as 20-60%.
The majority of the reported fistulas are grade A and management consists of continued drainage as most of these types of fistulas will heal without other intervention.
Occasionally a patient may need a percutaneous drain placed into a small fluid collection (grade B).
Rarely patients requiring ICU care for sepsis and multisystem organ failure of their pancreatic leak, and when recognized, need swift surgical intervention, which may end in completion pancreatectomy
Postoperative diabetes development after pancreatic surgery if a large portion of parenchyma is removed, and occurs less than 10% of the time (pancreatogenic diabetes).
Total pancreatectomy may also lead to liver steatosis, exocrine insufficiency with steatorrhea, retinopathy, increased neuropathy, and increased cardiovascular disease.
Splenic infarction can occur after ligation of the splenic vessels and can also result in gastric varices formation.
Delayed gastric emptying is a variable post-op complication that can occur in up to 50% of patients.
An anastomotic leak from the newly created anastomosis is a major cause of postoperative complications.
Intra Abdominal hemorrhage after pancreatic surgery can occur: early in the post-op course, it is likely due to the operative failure of hemostasis.
The later postoperative bleeding is seen with pancreatic leaks causing erosion into a vascular structure or causing a breakdown to an anastomotic site.
Pancreatectomy may be performed laparoscopically or with an open technique and is most often indicated in the presence of benign or malignant tumors, chronic pancreatitis, and pancreatic trauma.
