Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells.
Opsonins act as tags to label things that should be phagocytosed.
Opsonins are molecules that help facilitate phagocytosis, which is the process by which cells such as macrophages engulf and destroy pathogens like bacteria.
Opsonins coat the surface of pathogens, making it easier for immune cells to recognize and engulf them.
Common opsonins include antibodies and components of the complement system.
Targets of opsonins for phagocytosis, including: pathogens-bacteria, cancer cells, aged cells, dead or dying cells, excess synapses, or protein aggregates, such as amyloid plaques.
There are two main types of opsonin in blood that opsonised bacteria: complement proteins and antibodies.
There are at least 50 proteins that act as opsonins for pathogens or other targets.
Opsonins induce phagocytosis of targets by binding the targets such as bacteria, and then also binding phagocytic receptors on phagocytes.
Opsonins act as bridging molecules between the target and the phagocyte, bringing them into contact, and then usually activating the phagocytic receptor to induce engulfment of the target by the phagocyte.
All cell membranes have negative charges (zeta potential) which makes it difficult for two cells to come close together.
As opsonins bind to their targets they boost the kinetics of phagocytosis by favoring interaction between the opsonin and cell surface receptors on immune cells.
Opsonins override the negative charges from cell membranes.
It is important that opsonins do not tag healthy, non-pathogenic cells for phagocytosis.
Antibodies bind to antigens on the pathogen surface, enabling adaptive immunity.
Opsonins are related to the two types of immune systems: the adaptive immune and the innate immune systems.
Antibodies synthesized by B cells and are secreted in response to recognition of specific antigenic epitopes, and bind only to specific epitopic regions on an antigen.
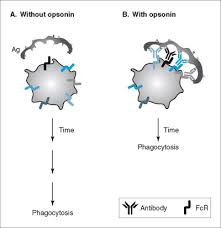
Antibody-mediated opsonization occurs when FcR on phagocytic cells recognize the Fc region of the antibody.
The adaptive opsonization pathway is composed of two fragments:antigen binding region (Fab region) and the fragment crystallizable region (Fc region).
The Fab region is able to bind to a specific epitope on an antigen, such as a specific region of a bacterial surface protein.
The Fc region of IgG is recognized by the Fc Receptor (FcR) on natural killer cells and other effector cells.
The binding of IgG to antigen causes a conformational change that allows FcR to bind the Fc region and initiate attack on the pathogen through the release of lytic products.
Antibody can tag tumor cells or virally infected cells, with NK cells responding via the FcR; this process is known as antibody-dependent cellular cytotoxicity (ADCC).
Both IgM and IgG undergo conformational change upon binding antigen that allows complement protein C1q to associate with the Fc region of the antibody.
C1q association recruits complement C4b and C3b, both of which are recognized by complement receptor 1, 3, and 4 which are present on most phagocytes: the complement system participates in the adaptive immune response.
The complement system, independently of the adaptive immune response, is able to opsonize pathogens before adaptive immunity may even be required.
Opsonins play a role in marking apoptotic cells for phagocytosis without a pro-inflammatory response.
C1q is capable of binding directly to apoptotic cells.
C1q activates complement, resulting in the cells being marked for phagocytosis by C3b and C4b.
C1q is an important contributor to the clearance of late apoptotic cells and debris.
Pathogens and other particles are marked by IgG antibodies by the innate immune system.
These antibodies interact with Fc receptors on macrophages and neutrophils resulting in phagocytosis.
The C1 complement complex can also interact with the Fc region of IgG and IgM immune complexes activating the classical complement pathway and marking the antigen with C3b.
C3b can spontaneously bind to pathogen surfaces through the alternative complement pathway. Furthermore, pentraxins can directly bind to C1q from the C1 complex.
