Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas
A chemical compound, an oxide of nitrogen with the formula N2O.
At room temperature, it is a colorless non-flammable gas, with a slightly sweet scent and taste.
At elevated temperatures, nitrous oxide is a powerful oxidizer similar to molecular oxygen.
Routes of administration Inhalation.
Metabolism 0.004%
Biological half-life 5 minutes
Excretion.
It is used uses, especially in surgery and dentistry, for its anaesthetic and pain-reducing effects.
Its euphoric effects upon inhaling it, a property that has led to its recreational use as a dissociative anaesthetic.
It is also used as an oxidizer in rocket propellants, and in motor racing to increase theoutput of engines.
Its atmospheric concentration reached 333 parts per billion (ppb) in 2020, increasing at a rate of about 1 ppb annually.
Nitrous oxide is a major scavenger of stratospheric ozone, with an impact comparable to that of chlorflluorohydrocarbons.
Being the third most important long-lived greenhouse gas, nitrous oxide also substantially contributes to global warming.
Nitrous oxide is used as a propellant, and has a variety of applications from rocketry to making whipped cream.
It is used as a recreational drug for its potential to induce a brief high.
It has potential for neurotoxicity and
potential to cause neurological damage.
Nitrous oxide may be used as an oxidizer in a rocket motor.
In vehicle racing, nitrous oxide allows the engine to burn more fuel by providing more oxygen during combustion, allowing the engine to produce more engine power.
Nitrous oxide is a strong oxidizing agent, roughly equivalent to hydrogen peroxide, and much stronger than oxygen gas.
Nitrous oxide is stored as a compressed liquid.
Liquid nitrous oxide increases engine power.
The gas is approved for use as a food additive, specifically as an aerosol spray propellant.
Its most common uses in this context are in aerosol whipped cream canisters and cooking sprays.
The nitrous oxide gas is extremely soluble in fatty compounds.
It produces whipped cream which is four times the volume of the liquid, whereas whipping air into cream only produces twice the volume.
Nitrous oxide also inhibits its degradation.
The whipped cream produced with nitrous oxide is unstable, and is not suitable for decorating food that will not be served immediately.
Cooking spray is made from various types of oils combined with lecithin, an emulsifier, may use nitrous oxide as a propellant.
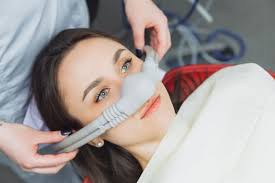
Nitrous oxide has been used in dentistry and surgery, as an anaesthetic and analgesic.
In hospitals by means of an automated relative analgesia machine, an anaesthetic vaporizer and a medical ventilator can deliver a precisely dosed and breath-actuated flow of nitrous oxide mixed with oxygen in a 2:1 ratio.
It is a weak general anaesthetic, and so is generally not used alone in general anaesthesia, but used mixed with oxygen for more powerful general anaesthetic drugs such as sevoflurane or desflurane.
Use in anaesthesia can increase the risk of postoperative nausea and vomiting.
Dentists use a machine which only delivers an N2O/O2 mixture for the patient to inhale while conscious.
Inhalation of nitrous oxide is used frequently to relieve pain associated with childbirth, trauma, oral surgery and acute coronary syndrome.
Use during labour has been shown to be a safe and effective aid for birthing women.
Fifty percent nitrous oxide can be considered for use by trained non-professional first aid responders in prehospital settings.
It is easy and safe to administer 50% nitrous oxide as an analgesic.
Recreational inhalation of nitrous oxide can cause euphoria and/or slight hallucinations.
Recreational use of nitrous oxide as a route has potential for causing neurological damage.
Nitrous oxide is a significant occupational hazard for surgeons, dentists and nurses.
Nitrous oxide is minimally metabolized with a rate of 0.004%.
Nitrous oxide retains its potency when exhaled into the room by the patient, and can pose an intoxicating and prolonged exposure hazard to the clinic staff if the room is poorly ventilated.
A continuous-flow fresh-air ventilation system or N2O scavenger system is used to prevent a waste-gas build up.
There is a set recommended exposure limit (REL) of 25 ppm (46 mg/m3) to escaped anaesthetic.
Exposure to nitrous oxide causes short-term decreased mental performance, audiovisual ability, manual dexterity, and induces spatial and temporal disorientation that could result in physical harm.
NO is neurotoxic
Long-term or habitual use of NO can cause severe neurological damage.
Nitrous oxide may cause neurotoxicity after extended exposure because of hypoxia.
In heavy or frequent users signs of peripheral neuropathy have been noted:
ataxia, paresthesias.
Occupational exposure to ambient nitrous oxide has been associated with DNA damage, impairing DNA synthesis.
Pure nitrous oxide inhalation without oxygen mixed in, this can eventually lead to oxygen deprivation resulting in loss of blood pressure, fainting and even heart attacks.
This can occur if the user inhales large quantities continuously, as with a strap-on mask connected to a gas canister.
Long-term exposure to nitrous oxide may cause vitamin B12 deficiency.
Use of nitrous oxide can cause serious neurotoxicity if the user has preexisting vitamin B12 deficiency.
NO inactivates the cobalamin form of vitamin B12 by oxidation.
Symptoms of vitamin B12 deficiency: including sensory neuropathy, myelopathy and encephalopathy, may occur within days or weeks of exposure to nitrous oxide anaesthesia in people with subclinical vitamin B12 deficiency.
Vitamin B12 levels should be checked in people with risk factors for vitamin B12 deficiency prior to using nitrous oxide anaesthesia.
NO directly modulates a broad range of ligand-gated ion channels.
Its anaesthetic, hallucinogenic and euphoriant effects are likely caused predominantly, or fully, via inhibition of NMDA receptor-mediated currents.
N2O may act to imitate nitric oxide (NO) in the central nervous system, and this may be related to its analgesic and anxiolytic properties.
Nitrous oxide is 30 to 40 times more soluble than nitrogen.
The effects of inhaling sub-anaesthetic doses of nitrous oxide:
Intoxication
Euphoria/dysphoria
Spatial disorientation
Temporal disorientation
Reduced pain sensitivity
Uncontrolled vocalisations and muscular spasms.
These effects generally disappear minutes after removal of the nitrous oxide source.
Low dose of N2O is an effective anxiolytic.
N2O’s anti-anxiety effect is associated with enhanced activity of GABAA receptors.
The analgesic effects of N2O are related to the interaction between the endogenous opioid system and the descending noradrenergic system.
Nitrous oxide, like morphine, interacts directly with the endogenous opioid system by binding at opioid receptor binding sites.
N2O-induced release of endogenous opioids causes disinhibition of brainstem noradrenergic neurons, which release norepinephrine into the spinal cord and inhibit pain signalling.
Nitrous oxide is a colourless, non-toxic gas with a faint, sweet odor.
Effects of the gas generally make the user appear stuporous, dreamy and sedated, while some in a state of euphoria, erupt in laughter.
Nitrous oxide is used in dentistry as an anxiolytic, as an adjunct to local anaesthetic.
Nitrous oxide is a minor component of Earth’s atmosphere and is an active part of the planetary nitrogen cycle.
Its concentration surpassed 330 ppb,and its growth rate of about 1 ppb per year has also accelerated during recent decades.
Nitrous oxide’s atmospheric abundance has grown more than 20% from a base level of about 270 ppb in year 1750.
It is estimated that about 29.5 million tonnes of N
2O (containing 18.8 million tonnes of nitrogen) were entering the atmosphere each year; of which 64% were natural, and 36% due to human activity.
Most of the N2O emitted into the atmosphere is from natural and anthropogenic sources.
N2O is produced by microorganisms such as denitrifying bacteria and fungi in soils and oceans.
Soils under natural vegetation are an important source of nitrous oxide, accounting for 60% of all naturally produced emissions.
Other natural sources include the oceans (35%) and atmospheric chemical reactions (5%).
The main components of anthropogenic emissions are fertilized agricultural soils and livestock manure (42%), runoff and leaching of fertilizers (25%), biomass burning (10%), fossil fuel combustion and industrial processes (10%), biological degradation of other nitrogen-containing atmospheric emissions (9%) and human sewage (5%).
Agriculture enhances nitrous oxide production through soil cultivation, the use of nitrogen fertilizers and animal waste handling.
Such processes stimulate naturally occurring bacteria to produce more nitrous oxide.
Natural processes that generate nitrous oxide may be classified as nitrification and denitrification.
Nitrification and denitrification are affected by soil chemical and physical properties such as the availability of mineral nitrogen and organic matter, acidity and soil type, as well as climate-related factors such as soil temperature and water content.
Nitrous oxide has significant global warming potential as a greenhouse gas.
On a per-molecule basis, considered over a 100-year period, nitrous oxide has 265 times the atmospheric heat-trapping ability of carbon dioxide (CO): because of its low concentration (less than 1/1,000 of that of CO2, its contribution to the greenhouse effect is less than one third that of carbon dioxide, and also less than water vapour and methane.
38% or more of the N2O entering the atmosphere is the result of human activity, control of nitrous oxide is considered part of efforts to curb greenhouse gas emissions.
Nitrous oxide is released into the atmosphere through agriculture, when farmers add nitrogen-based fertilizers onto the fields, and through the breakdown of animal manure.
Approximately 79 percent of all nitrous oxide released in the United States came from nitrogen fertilization.
Nitrous oxide is also released as a by-product of burning fossil fuels.
It is also emitted through the manufacture of nitric acid, which is used in the synthesis of nitrogen fertilizers.
The production of adipic acid, a precursor to nylon and other synthetic clothing fibres, also releases nitrous oxide.
The total amount of nitrous oxide released that is of human origins is about 40 percent.
Nitrous oxide has also been implicated in thinning the ozone layer.
