A surgical procedure for implanting a neurostimulation device to treat obstructive sleep apnea (OSA).
The Inspire® Upper Airway Stimulation (IUAS) device is used to treat patients with moderate to severe sleep apnea who are unable to use CPAP.
Originally, the implantation procedure required three incisions, but the FDA has now approved a two-incision implant procedure, which reduces procedure time and pain for the patient.
In addition to moderate to severe apnea, other criteria for IUAS include an apnea-hypopnea index between 15 and 65, BMI in the 32-35 range, less than 25% central apneas and age 18 and above.
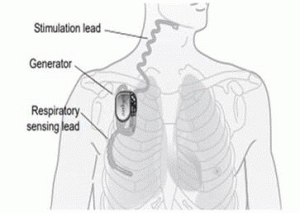
The IUAS consists of three parts —the implantable pulse generator (IPG), a stimulation lead and a sensing lead.
Outpatient surgery requires three separate incisions in order to implant the device.
One incision is made in the upper neck to place the stimulation lead around the hypoglossal nerve, and another incision is made in the upper chest to place the IPG.
The third incision is made over the lateral chest to place the sensor between the 5th and 6th ribs.
The activated device works by sensing the patient’s respiration and creating a coordinated stimulation of the tongue.
This stimulation stiffens and protrudes the tongue slightly, which results in opening the airway while sleeping.
A more efficient surgical approach is a two-incision approach, the intermittent pulse generator and the sensor are now placed through the same incision.
The sensor is placed deep onto the pectoralis major muscle between the second and third ribs in the anterior chest.
The success rate of the therapy is between 70 and 80%.
