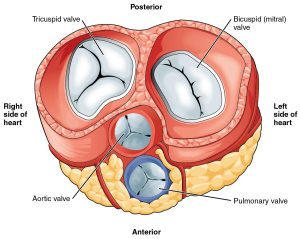
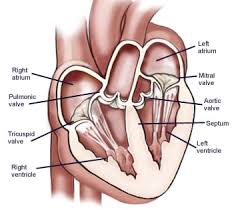
 The mitral valve and subvalvular apparatus includes the annulus, the valve leaflets, chordae tendinae, papillary muscles and the left ventricle wall.
The mitral valve and subvalvular apparatus includes the annulus, the valve leaflets, chordae tendinae, papillary muscles and the left ventricle wall.
Degenerative disease most common cause of organic mitral valve disease in the US.
Mitral valve disease incidence 2-3% of individuals.
Mitral valve disease prevalence in the aged is more than 10% of those older than age 75 years.
Mitral valve prolapse most common functional abnormality associated with degenerative mitral valve disease.
Mitral valve prolapse defined on echocardiography as systolic atrial displacement of the mitral valve so that it extends above the annulus by a minimum of 2mm.
Mitral valve prolapse associated with leaflet redundancy and chordal elongation.
Mitral valve disease classically may be associated with thickened and redundant myxomatous mitral leaflets with abnormal connective tissue.
Thickened mitral leaflets associated with extracellular matrix changes produced by overexpressed catabolic enzymes (Liu AC).
The valve has anterior and posterior leaflets.
Each leaflet consists of three segments, designated P1, P2 and P3 in the posterior leaflet and A1, A2, and A3 in the anterior leaflet.
The valve leaflets receive chordae tendinae from the anteriolateral and posteriomedial papillary muscles.
Competence of the valve relies on the interaction of the valve and the sub valvular apparatus.
The papillary muscles contract during systole and increases the tension on the chordae tendinae and prevent the valve leaflets from everting into the left atrium.
Mitral valve calcification will develop in 50% of patients with ESRD.
Polypoid calcification deposits occur in the anterior and posterior leaflet bases, sparing the free edges and chordae tendinae.
Senile degenerative mitral valve calcification is associated with small nodules or ridges posteriorly, and rarely affects the anterior leaflet.
Mitral regurgitation is divided into primary disease with structural or degenerative abnormality of the mitral valve or secondary pdisease of the left ventricle, which interferes with the function and integrity of mitral apparatus.
Mitral regurgitation leads to left atrial and left ventricular dilatation.
Mitral regurgitation that occurs acutely is associated with rapid increase in left atrial pressure with secondary increases in pulmonary venous pressures, with pulmonary edema.
Gradual onset of mitral regurgitation results in slow left atrium dilatation and little increase in atrial pressure and is associated with predispositon to the development of atrial fibrillation.
Mitral regurgitation associated with left ventricular volume increase to maintain forward stroke volume, and is associated with a normal or high left ventricular ejection fraction.
With mitral regurgitation left ventricular remodeling occurs and may result in reduced left ventricular function.
Most common cause of severe regurgitation is due to ruptured elongated chordae.
Rupture of elongated chordae results in flailing and unsupported leaflets.
Rupture of elongated chordae can lead to severe mitral regurgitation in 12% of patients over 1.5 years of follow-up (EnriqueSarano M).
Rupture of elongated chordae most common in men and elderly.
Increasing severity of mitral regurgitation associated with increased risk of congestive heart failure, atrial fibrillation and death.
Death rate due to cardiovascular causes, from asymptomatic moderate mitral regurgitation, or a LVEF of less than 50% is greater than 3% per year (Avierinos JF).
Mitral valve surgery is used to treat both primary and secondary MR.
Surgery, whether mitral valve repair or replacement, has been the standard of care in treating patients with primary (degenerative) MR.
Patients with secondary MR are often treated medically, but surgery is also often employed,
Repair compared to mitral valve replacement with a prosthetic valve achieves better survival and equivalent, if not better durability.
Following successful mitral valve repair, patients who maintain sinus rhythm do not need long-term anticoagulant therapy, and can resume full activities
Early mitral valve repair of leaflet prolapse prevents severe manifestations of untreated mitral regurgitation.
Mitral valve repair using robotic assistance improves the return to normal activity in otherwise healthy asymptomatic individuals.
Mitral valve repair, robotically assisted, can be performed with zero mortality, a high success rate for leaflet prolapse.
Currently, surgical mitral valve repair is the standard criteria for degenerative disease with excellent outcomes in terms of survival, freedom from recurrent severe mitral regurgitation, and freedom from re-operation.
Following successful robot assisted mitral ventricular repair, left ventricle size and volume regression occur almost immediately.
Robotically assisted repair in experienced centers has advantages including smaller incisions, shorter length of stay, and potentially higher technical precision.
Delaying mitral valve repair in patients with severe mitral regurgitation is harmful and allows LVEF to decrease below 60%, and permits expansion of left ventricular and systolic diameter beyond 40 mm.
With mitral valve regurgitation awaiting onset of symptoms increases early and late mortality after surgical correction.
Asymptomatic patients who undergo mitral valve repair have improved progression of LV dimensions, recovery of normal LVEF with time.
Mitral valve repair is superior over valve replacement for mitral leaflet prolapse.
Triangular resection of a prolapsed posterior leaflet can effectively eliminate prolapse and restore normal leaflet coaptation.
Anterior leaflet prolapse can be repaired with artificial neochord resuspension.
Mitral valve repairs are generally supported with a annuloplasty device.
Mitral annular dilatation occurs mainly in the posterior two thirds of the mitral annulus in degenerative disease and reduction and stabilization using a annuloplasty band improves safety, effectiveness and durability
Minimally invasive mitral valve repair decreases postoperative pain, is associated with shorter postoperative ventilation time, fewer blood transfusions, improve respiratory function, equivalent or diminished postoperative length of hospitalization, excellent safety and durability.
Robotic assistants improves outcomes.
Cardioplegic arrest during the mitral valve repair provides a bloodless field.
Robot assisted mitral valve repair has a median hospital stay of three days, and a complication rate as low as, oral lower than, traditional median sternotomy procedures.
In a retrospective study among patients 50-69 years undergoing mitral valve replacement mechanical prosthetic versus bioprosthetic replacement: there was no significant survival difference in 15 years (Chikwe J et al).
In the study mechanical prosthetic valves were associated with the lower risk of reoperation but a greater risk of bleeding and stroke.
Bioprosthetic valves are recommended in patients older than seventy years in whom the likelihood of needing reoperation for bioprosthetic valve degeneration is low.
Bio prosthetic structural valve generation is accelerated in younger patients who therefore face a much higher lifetime risk of reoperation.
Preoperation a much lower for mechanical prosthetic valves, associated with increased thromboembolic and hemorrhagic complications, and lifestyle limitations because of lifelong anticoagulation.
Mitral stenosis is usually due to rheumatic heart disease, but heavy calcifications of the mitral annulus rwith extension into the leaflets may cause obstruction the left ventricular inflow particularly in the elderly.
Treatment of mitral valve disorders is dependent on the underlying cause, pathophysiology, and natural history of the process.
