 Infantile respiratory distress syndrome (IRDS), also called respiratory distress syndrome of newborn, or increasingly surfactant deficiency disorder (SDD),and previously called hyaline membrane disease (HMD)
Infantile respiratory distress syndrome (IRDS), also called respiratory distress syndrome of newborn, or increasingly surfactant deficiency disorder (SDD),and previously called hyaline membrane disease (HMD)
In premature infants caused by developmental insufficiency of pulmonary surfactant production and structural immaturity in the lungs.
It can also be a consequence of neonatal infection and can result from a genetic problem with the production of surfactant-associated proteins.
IRDS affects about 1% of newborns and is the leading cause of death in preterm infants.
Elective caesarean sections increased the incidence of respiratory distress in term infants.
The incidence decreases with advancing gestational age, from about 50% in babies born at 26–28 weeks to about 25% at 30–31 weeks.
The IRD syndrome is more frequent in males, Caucasians, infants of diabetic mothers and the second-born of premature twins.
Neonates with respiratory failure are commonly born prematurely at risk for intracranial hemorrhage, and with immature lungs require weeks or months of respiratory support, placing them at risk for subsequent neurodevelopment impairment.
IRDS begins shortly after birth and is manifested by tachypnea (more than 60 breaths per minute), a fast heart rate, chest wall retractions, expiratory grunting, nasal flaring, and blue discoloration of the skin.
As the disease progresses, the baby may develop ventilatory failure.
IRDS remains the most common single cause of death in the first month of life in the developed world.
Complications include metabolic disorders of acidosis, hypoglycemia, patent ductus arteriosus, low blood pressure, chronic lung changes and bleeding in the brain.
Prematurity has its additional effect on other organ functions.
The characteristic histopathology seen in babies who die from RDS is waxlike layers of hyaline membrane line the collapsed alveoli of the lung.
Theblungs show bleeding, overdistention of airways, and damage to the lining cells.
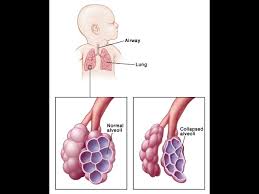
The lungs in respiratory distress syndrome are developmentally deficient in surfactant.
Surfactant helps prevent the collapse of the terminal air spaces, the future site of alveolar development, throughout the normal cycle of inhalation and exhalation.
Surfactant deficiency is related to inhibition from the insulin that is produced in the newborn, especially those of diabetic mothers.
Pulmonary surfactant is a complex system of lipids, proteins and glycoproteins that is produced in specialized lung cells called Type II cells or Type II pneumocytes.
The surfactant is packaged by the cell in structures called lamellar bodies, and extruded into the air spaces.
The lamellar bodies then unfold into a complex lining of the air space.
This layer reduces the surface tension of the fluid that lines the alveolar air space.
Surface tension is responsible for approximately 2/3 of the inward elastic recoil forces.
By reducing surface tension, surfactant prevents the air spaces from completely collapsing on exhalation.
The decreased surface tension allows reopening of the air space with a lower amount of force.
Without adequate amounts of surfactant, the air spaces collapse and are very difficult to expand.
A pulmonary surfactant-deficient lung is characterized by collapsed air spaces alternating with hyperexpanded areas, vascular congestion, and, eventually hyaline membranes.
Hyaline membranes are composed of fibrin, cellular debris, red blood cells, rare neutrophils and macrophages.
Hyaline membranes appear as an eosinophilic, amorphous material, lining or filling the air spaces and blocking gas exchange: unable to pick up oxygen and unload carbon dioxide.
As a result blood oxygen levels fall and carbon dioxide rises, resulting in acidosis and hypoxia.
Diagnosis
The diagnosis is made by the clinical picture and the chest X-ray, which demonstrates decreased lung volumes, absence of the thymus, a ground glass appearance or diffuse airspace and interstitial opacities affecting all lobes of the lung and air-bronchograms.
Prevention
Maternal glucocorticoids for very premature deliveries speeds the production of surfactant.
Antenatal glucocorticoid treatment is recommended for women at risk for preterm delivery prior to 34 weeks of gestation.
In pregnancies of longer than 30 weeks, the fetal lung maturity may be tested by sampling the amount of surfactant in the amniotic fluid by amniocentesis.
Tests are available that correlate with the production of surfactant: the lecithin-sphingomyelin ratio (“L/S ratio”), the presence of phosphatidylglycerol (PG), and the surfactant/albumin (S/A) ratio.
The S/A ratio, the result is given as milligrams of surfactant per gram of protein:
For the L/S ratio, if the result is less than 2:1, the fetal lungs may be deficient in surfactant: A S/A ratio less than 35 indicates immature lungs, between 35-55 is indeterminate, and greater than 55 indicates mature surfactant production.
The presence of PG usually indicates fetal lung maturity.
Despite only 1% of all birth complications being attributed to respiratory distress syndrome, there is a significantly higher prevalence in prematurely born babies.
Incidence rates of IRDS in premature infants born at 30 weeks of gestational age (GA) is at 50%.
Incidence rate of IRDS is up to 93% for infants born prematurely at 28 weeks of gestational age or younger.
It can be diagnosed within hours of delivery.
IRDS usually leads to morbidity and mortality in preterm infants.
Common risks factors: male gender, white race, late preterm delivery, maternal diabetes, perinatal hypoxia and ischemia and low birth weight.
The incidence rate of IRDS for 24 weeks was 98%, for 34 weeks the incidence is 5%, and for 37 weeks the incidence rate was less than 1%.
The results demonstrate that the incidence of IRDS increases with decreasing age at birth.
TREATMENT:
Oxygen is given with a small amount of continuous positive airway pressure (CPAP), and intravenous fluids are administered to stabilize the blood sugar, blood salts and blood pressure.
CPAP is associated with a reduction in respiratory failure, mechanical ventilation and mortality: it is associated with an increased rate of pneumothorax compared to spontaneous breathing with or without supplemental oxygen.
Mechanical ventilation may be required.
Exogenous preparation of pulmonary surfactant, either synthetic or extracted from animal lungs, is given through the endotracheal tube into the lungs.
Surfactant use can decrease the risk of death for very low-birth-weight infants who are hospitalized by 30%.
Chronic lung disease, including bronchopulmonary dysplasia, is common in severe RDS.
The mortality rate for babies greater than 27 weeks of gestation is less than 20%.
Extracorporeal membrane oxygenation (ECMO) treatment, cannot be used if the newborn is under 4.5 pounds (2 kg), because they have extremely small vessels for cannulation.
In infants aged less than 34 weeks of gestation, several physiologic systems are not well-developed: cerebral vasculature and germinal matrix, resulting in high sensitivity to slight changes in pH, PaO2 and intracranial pressure.
Preterm infants are at unacceptably high risk for intraventricular hemorrhage (IVH) if administered ECMO at a gestational age of less than 32 weeks.
The INSURE method, is an effective approach to managing preterm neonates with respiratory distress, decreasing
the use of mechanical ventilation and lowers the incidence of bronchopulmonary dysplasia (BPD).
Preterm infants requiring surfactant replacement treated using the INSURE technique, which requires sedation and comprises tracheal intubation, surfactant instillation and extubation.
Lung-maturity tests: the microbubble test, lamellar body counts and measurements of lecithin-sphingomyelin ratio (L/S)[30] with chemometrics.
The routine use of sedatives and opioid pain killers is not recommended for newborn infants who require mechanical ventilation to breathe.
Among children who survive respiratory failure and discharge, still have significantly lower IQ scores compared with matched siblings.
