 Also called hepatitis delta virus.
Also called hepatitis delta virus.
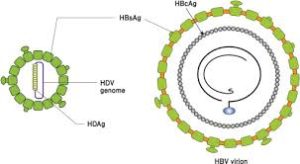
A small RNA virus that depends on the hepatitis B virus for entry into hepatocytes, and for assembly and secretion of newly formed virions.
A defective, hypotropic pathogenic agent.
Prevalence unknown but estimated between 62 and 72 million people worldwide are infected with HDV, representing 1% of the global population.
It is the most severe form of chronic viral hepatitis and is associated with a risk of hepatocellular carcinoma that is 2 to 6 times as high, and a risk of death that is 2 to 3 times as high as the risks associated with hepatitis B virus monoinfection.
HDV is endemic in Mongolia, other parts of Asia, Africa, southern and eastern Europe, the Middle East, and Brazil.
RNA virus is replication defective and can cause infection only when it is encapsulated by HBsAg.
HDV only infects people who are also infected by HBV.
HDV is a satellite RNA virus that requires hepatitis B virus surface antigen for entry into hepatocytes and for propagation.
I HDV, is transmitted by the parental route through infectious body fluids.
IV drug uses are at highest risk for infection, because of contaminated syringes.
Prevalence in 13 to 14.6% of HBV carriers.
It is estimated to affect between 10 and 20 million persons worldwide.
In the US 5 to 10% of persons positive for HBsAG are also seroprevalence positive anti-HDV antibody which means that HDV infection meets threshold for designation of an orphan disease, occurring in less than 200,000 persons.
High risks in intravenous drug users and those with high risk sexual behavior.
Its prevalence is decreasing as the worldwide implementation of HPV vaccination programs grow, reducing the number of carriers who are susceptible to HDV.
Because of HPV vaccination, there is now an age related prevalence shift to older persons.
It is the most severe and progressive form of viral hepatitis in humans.
HDV is the smallest viral pathogen infecting humans.
It is a defective virus because it does not encode its own envelope proteins and requires the presence of HBV for virion assembly, replication, and transmission.
There are eight genotypes, and has a circular, single-stranded RNA of approximately 700 nucleotides.
Patients infected with HDV always have a simultaneous HBV infection either acquired as an acute co-infection or as a superinfection with chronic HBV.
HDV infection may occur as an acute coinfection with HBV when both viruses are acquired together or as a superinfection, when HBV infects a person who has chronic HBV infection.
An acute HDV-HBV coinfection is followed by clearance of both viruses in approximately 95% of patients, whereas more than 90% of HDV superinfections, result IN chronic HDV – HBV infection.
Long term coinfection with HBV & HDV is considered to be the most serious type of chronic viral hepatitis.
Compared to monoinfection with HBV, HBV-HDV, coinfection accelerates the course of disease, increases the risk of cirrhosis, hepatocellular carcinoma, need for early transplant, and early death.
It is dependent on the genetic information from HBV for its multiplication and causes hepatitis only in the presence of HBV.
A single-stranded, circular RNA, which is the smallest virus known to infect humans.
Occurs with acute coinfection with HBV, with the latter established first to provide the HBsAg needed for development of complete hepatitis D virions.
Can occur as a super infection of a chronic carrier of HBV with a new inoculum of HDV and results in disease 30-50 days later.
The coinfection with HBV results in hepatitis process that ranges from mild to fulminant disease.
Fulminant disease is more likely with coinfection of D and B hepatitis than with HBV alone.
Clinically indistinguishable from acute HBV infection.
Acute HDV-HBV coinfection may manifest as acute hepatitis, typically followed by clearance of both viruses, although the risk of acute liver failure is higher in patients with confections in patients with acute HBVmonoinfection.
HDV superinfection may manifest as acute hepatitis in patients with previously undiagnosed chronic HBV infection or as an exacerbation of chronic liver disease in patients with known chronic hepatitis B.
HDV infection is transmitted through parenteral exposure – injection drug use, needlestick, injuries, or sexual exposure.
HDV infection is associated with an increased risk of acute liver failure, especially in intravenous drug users.
HDV interferes with HBV replication, therefore most patients with chronic hepatitis D have low serum, HPV-DNA levels.
Chronic hepatitis D has an accelerated course with rapid progression to cirrhosis, high incidence of liver related mortality, and hepatocellular carcinoma than chronic, hepatitis B infection alone.
More than 50% of people with chronic hepatitis D have a liver related mortality within 10 years.
Approximately 90% of patients with acute HDV – HBV co-infection clear their infection spontaneously, but the acquisition of HDV as a superinfection typically results in a chronic HDV infection.
Chronic HDV infection has a viremia that lasts more than six months and frequently results in a severe clinical course.
Chronic HDV-HBV coinfection progresses to cirrhosis in 70 to 80% of cases within 5 to 10 years.
Chronic HDV infections are associated with a 3 to 6 fold increase in hepatocellular carcinoma, compared to HBV.
Diagnosis:
The diagnosis involves specific serological testing-the presence of hepatitis D virus antibody.
Acute HDV-HBV coinfection is diagnosed by the simultaneous presence of HBsAg and hepatitis B core antibody, and HDV RNA.
All HBsAG positive persons should be tested.
Testing should be done only in patients who are HBsAG positive.
Persistent severe HD viremia is the most important risk factor for progression and chronic hepatitis C, and is almost invariably severe.
Treatment goals include improvement in clinical outcomes by preventing cirrhosis, hepatic decompensation, hepatoma, and liver related mortality by inhibiting hepatitis D viral replication and decreasing hepatic inflamation and fibrosis.
Treatment for chronic HDV is a 12 month course of Peglated interferon: sustained responses occur in only 25% and relapse rates are high.
Oral anti-viral drugs for the treatment of HBV infection, are effective as monotherapy in suppressing HBV replication, but they do not effectively suppress HDV replication.
Currently, there are no approved therapies for hepatitis D.
Interferon alpha and long acting formulations pegylated interferon alpha inhibit HD replication, decrease liver inflammation, and fibrosis, and improves survival but these effects are durable only in 30% of treated patients.
Bulevirtude in a randomized phase 3 trial for chronic hepatitis D reduced HDV RNA levels and ALT levels in patients with chronic hepatitis D.
The combination of bulevirtude plus peg interferon alpha 2a is superior tobulevirtude mono therapy with regard to undetectable HD RNA level at 24 weeks after the end of treatment.
Lonaformib has been shown to decrease HDV RNA levels.
The optimal endpoint for treatment is the loss of HBsAG, which is only a rare achievement.
HBV vaccine prevents both HBV and HDV infection, but there is no vaccine to prevent HDV infection in persons already infected with HBV.
HBV nucleoside/nucleotide analogues are used in patients with chronic hepatitis D, who have indications for HBV treatment for cirrhosis and high levels of HBVDNA and elevated ALT or liver fibrosis.

One reply on “Hepatitis D (HDV)h”
It is very good information about some myths regarding the curing of both HIV and hepatitis C infection. It was somewhat useful for many including me. It is a must-read blog to explore the new info about HIV and hepatitis C infection curable medicines. Keep it posting these kinds of informative blogs in the future!