 The glymphatic system or glymphatic clearance pathway, or paravascular system is a system for waste clearance in the central nervous system (CNS).
The glymphatic system or glymphatic clearance pathway, or paravascular system is a system for waste clearance in the central nervous system (CNS).
The name glymphatic system depends upon glial cells and it functions similarly to the peripheral lymphatic system.
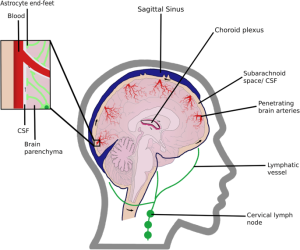
Cerebrospinal fluid (CSF) flows into the paravascular space around cerebral arteries, combining with interstitial fluid (ISF) and parenchymal solutes, and exiting down venous paravascular spaces.
The glymphatic pathway consists of a para-arterial influx route for CSF to enter the brain parenchyma, coupled to a clearance mechanism for the removal of interstitial fluid (ISF) and extracellular solutes from the interstitial compartments of the brain and spinal cord.
Exchange of solutes between CSF and interstitial fluid is driven primarily by arterial pulsation.
The exchange of solutes is regulated during sleep by the expansion and contraction of brain extracellular space.
The clearance of soluble proteins, waste products, and excess extracellular fluid is accomplished through convective bulk flow of interstitial fluid facilitated by astrocytic aquaporin 4 (AQP4) water channels.
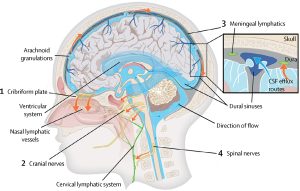
The dural sinuses and meningeal arteries are lined with conventional lymphatic vessels, and that this vasculature forms a connecting pathway to the glymphatic system.
Perivascular spaces are CSF-filled channels formed between the brain blood vessels and leptomeningeal sheathes that surround cerebral surface vessels and proximal penetrating vessels.
Around these penetrating vessels, perivascular spaces take the form of Virchow-Robin spaces.
Where the Virchow-Robin spaces terminate within the brain parenchyma,perivascular CSF can continue traveling along the basement membranes surrounding arterial vascular smooth muscle, to reach the basal lamina surrounding brain capillaries.
CSF movement along these paravascular pathways is rapid and arterial pulsation is suspected to be an important driving force for perivascular fluid movement.
When cerebral arterial pulsation was either increased or decreased, the rate of perivascular CSF flux in turn increases or decreases, respectively.
Astrocytes extend long processes that interface with neuronal synapses, and their end-feet projections completely ensheathe the brain’s entire vasculature.
Astrocytes facilitate changes in blood flow and play a role in waste removal in the brain.
Astrocytes express water channels called aquaporins, which are membrane-bound channels that play critical roles in regulating the flux of water into and out of cells.
The presence of aquaporins in biological membranes facilitates a 3– to 10-fold increase in water permeability.
Two types of aquaporins are expressed in the CNS: aquaporin-1, expressed by specialized epithelial cells of the choroid plexus, and aquaporin-4 (AQP4), which is expressed by astrocytes.
Aquaporin-4 expression in astrocytes is highly polarized to the endfoot processes ensheathing the cerebral vasculature.
Up to 50% of the vessel-facing endfoot surface that faces the vasculature is occupied by arrays of AQP4.
AQP4 is essential for paravascular CSF–interstitial exchange.
Based upon this role of AQP4-dependent glial water transport in the process of paravascular interstitial solute clearance, this brain-wide glio-vascular pathway the glymphatic system.
Clearance of interstitial waste products increases during the resting state.
The changes in efficiency of CSF–ISF exchange between the awake and sleeping brain are caused by expansion and contraction of the extracellular space, which increased by ~60% in the sleeping brain to promote clearance of interstitial wastes such as amyloid beta.
It is hypothesized that the restorative properties of sleep may be linked to increased glymphatic clearance of metabolic waste products produced by neural activity in the awake brain.
Glymphatic activity decreases sharply during aging.
The brain’s system of perivascular pathways plays an important role in transporting small lipophilic molecules.
Perivascular transport of lipids through the glymphatic pathway activate glial calcium signalling.
Depressurization of the cranial cavity, impairs glymphatic circulation, and leads to unselective lipid diffusion, intracellular lipid accumulation, and pathological signalling among astrocytes.
The failure of the glymphatic system in aging might thus contribute to the accumulation of misfolded and hyperphosphorylated proteins and thereby render the brain more vulnerable to developing a neurodegenerative pathology or perhaps escalate the progression of cognitive dysfunction.
Neurodegenerative diseases such as amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease are all characterized by the progressive loss of neurons, cognitive decline, motor impairments, and sensory loss: proteinopathies due to the common assemblage of misfolded or aggregated intracellular or extracellular proteins.
It is suspected that in the amyloid hypothesis of Alzheimer’s disease, the aggregation of amyloid-beta, a peptide normally produced in and cleared from the healthy young brain, into extracellular plaques drives the neuronal loss and brain atrophy that is the hallmark of Alzheimer’s dementia.
The extent of the involvement of the glymphatic system in Alzheimer’s disease and other neurodegenerative disorders remains unclear.
The glymphatic system may be impaired following acute brain injuries such as ischemic stroke, intracranial hemorrhage, or subarachnoid hemorrhage.
The glymphatic system may also be involved in the pathogenesis of amyotrophic lateral sclerosis.
Downstream of the glymphatic system’s waste clearance from the ISF to the CSF, the meningeal lymphatic system drains fluid from the glymphatic system to the meningeal compartment and deep cervical lymph nodes.
