 Carbohydrate intolerance of varying degrees of severity, with onset or first recognition during pregnancy.
Carbohydrate intolerance of varying degrees of severity, with onset or first recognition during pregnancy.
Diabetes develops in roughly 6% to 7% of pregnancies, with approximately 90% attributable to gestational diabetes.
Diabetic ketoacidosis (DKA) in a pregnant woman is life-threatening caused by an absolute or relative deficiency in insulin.
Screening and treatment for gestational diabetes at 24 to 28 weeks gestation is recommended.
Women with pregnancies, complicated by gestational hyperglycemia at less than 20 weeks gestation show accelerated fetal growth by 24 to 28 weeks gestation and have a great at perinatal mortality than women who receive a diagnosis of gestational diabetes later in pregnancy.
There is a linear relationship between fasting glucose levels in early pregnancy and adverse outcomes.
There is a continuous, does dependent association with hyperglycemia and adverse pregnancy outcomes: primary C-section, preterm birth, hypertensive disorders of pregnancy, and infant associated outcomes of large for gestational age or birth weight equal to or greater than 90th percentile, hyperbilirubinemia, hypoglycemia, and need for neonatal ICU.
Diabetic ketoacidosis is a ketogenic state and may occur even at normal blood glucose levels.
Patients with type one diabetes are at greatest greatest risk for DKA, but DKA may occur in pregnancy with any type of diabetes.
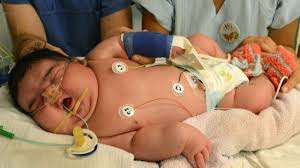
One in two babies born to women with type one diabetes have complications, most commonly preterm birth, large birth weight, and admission to the neonatal intensive care unit.
Antenatal maternal hyperglycemia is the most important risk factor for complications in newborns, with the highest risk among persons who begin their pregnancy with above target glycated hemoglobin levels.
Gestational diabetes is diagnosed in the second or third trimester of pregnancy in the absence of overt diabetes before gestation.
Pre-existing diabetes complicates 0.9% of pregnancies in the US and increases the risk of adverse maternal and neonatal outcomes.
Patients with gestation diabetes have a 30% increase risk of Cesarean delivery, high rates of induced labor, and a 50% increase in gestational hypertension: offspring have a 70% increase risk of prematurity and are 30% more likely to be large for gestational age.
Large for gestational age babies have an increased risk of operative death, shoulder dystocia, and birth injuries along with lung disease, jaundice, and hypoglycemia.
About 1/3 of patients affected by gestational diabetes experience adverse outcome including: cesarean delivery, preeclampsia, severe maternal morbidity, which includes transfusion and material ICU admission.
Prevalence of GDM is higher among women of African American, Hispanic, Native American, Asian, and Pacific Islander descent.
Specific risks of uncontrolled diabetes in pregnancy include preeclampsia, congenital defects, preterm delivery, macrosomia, and stillbirth.
The risk of major birth defects is 6-10% in women with pre-gestational diabetes with HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10.
In type one diabetes 50% of infants experience complication such as prematurity, large for gestational age, admission to a neonatal intensive care unit.
Exposure to all forms of diabetes in pregnancy confers a higher risk of childhood adiposity, insulin resistance, and adverse neurodevelopmental outcomes.
Gestational diabetes exposes the unborn baby to an abnormal metabolic environment with excessive nutrient availability, leading to fetal overgrowth.
During pregnancy there is insulin resistance reducing insulin dependent glucose uptake in muscles and fat and serves as a maternal physiological adaption to preserve carbohydrates for the rapidly growing fetus.
Immediate treatment of gestational diabetes before 20 weeks gestation leads to a modestly, lower incidence of adverse neonatal outcomes then no immediate treatment.
The impaired insulin mediated suppression of maternal lipolysis and fat oxidation provides fatty acids as an alternative energy source.
These factors plus increased progesterone, estrogen, cortisol, human placental growth hormone require a 2 to 3 fold increase in insulin production to meet this challenge.
Patients with GDM have a 7-fold increased risk for developing type 2 diabetes later in life and require lifelong screening for diabetes.
About 50% of women with GDM go on to develop type 2 DM, on average between 22 and 28 years after pregnancy.
Up to 60% of Latin-American women may develop type 2 diabetes 5 years after being diagnosed with GDM.
Standard treatment is diet and in some cases insulin during pregnancy.
Women with gestational diabetes have a considerable risk of having diabetes later in life.
Insulin sensitivity decreases sharply in the second and early third trimesters of pregnancy reducing glucose uptake in tissues such as muscle and fat and serves as a maternal physiological adaptation to preserve carbohydrates for the rapidly growing fetus.
Insulin secretion increases significantly by early pregnancy, even before increases in insulin resistance.
However, the insulin secretary response is often inadequate and hyperglycemia develops.
As many as 70% of women with gestational diabetes will develop type II diabetes by 10 years after delivery (Kim C).
Among parous women with type 2 DM, approximately one-third had a history of gestational DM.
An indicator of the presence of type II diabetes and not type I diabetes.
Associated with increased maternal and perinatal morbidity and increased perinatal mortality.
Complications include neonatal hypoglycemia and maternal hypertension.
Fetal exposure to diabetes during pregnancy can lead to long-term developmental changes that manifest in higher rates of diabetes and obesity in adulthood, adverse cardiometabolic profiles, a greater risk of hospitalizations, medication use, and mortality.
Diabetes in pregnancy may have an influence on neurodevelopmental outcomes with offspring of mothers with diabetes having a lower long-term cognitive function, worse school performance, heightened risk of autism, increased attention deficit/hyperactivity disorder compared with offspring of mothers without pre-existing diabetes.
Diabetic control improves perinatal outcomes.
15% to 47% of such pregnancies are complicated by macrosomia ( 3 to 6 fold increase).
Greater increase in the frequency of shoulder dystocia and other maternal-fetal complications with increasing glucose levels during an oral glucose tolerance test.
Complicates between 3% and 5% of all pregnancies and is associated with an increase in perinatal morbidity.
GDM is associated with approximately a tenfold higher lifetime risk of type two diabetes.
Offspring of mothers with pre-existing diabetes or gestational diabetes are heavier at birth and at every age with an increase risk of type two diabetes compared to mothers born without diabetes.
Type one diabetes risk is increased in offspring with maternal or paternal diabetes of any type, and appears even higher with paternal diabetes.
Women with type two diabetes have less dramatic changes in glucose metabolism and are less prone to diabetic ketoacidosis and cesarean delivery compared with those with type one diabetes.
Associations of milder forms of gestational diabetes with adverse outcomes is unclear.
The Hyperglycemia and Adverse Pregnancy outcome (HAPO) study described a strong continuous association between maternal glucose concentration, increased cord blood, increasing birth weight, increase serum C-peptide and other markers of perinatal complication.
In the HAPO study children of mothers with geststational diabetes versus those without it showed no difference in childhood overweight for obesity defined by BMI index at a median folloup of 11.4 years (Lowe WL).
When diagnosed by 26 weeks associated with an increased risk of autism spectrum disorders.
The Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) a randomized trial for the treatment of gestational diabetes suggested that treatment reduced serious perinatal complications.
ACHOIS trial indicated that treatment does not reduce rates of symptomatic neonatal hypoglycemia, or jaundice requiring photo therapy.
ACHOIS trial indicated treatment reduced rate of shoulder dystocia when women with mild gestational diabetes were treated compared to untreated patients, 1% vs. 3%.
Increased birth weight and neonatal fat results in increased risk of impaired glucose tolerance and childhood obesity.
In the randomized trial of 958 women with mild gestational diabetes treatment did not significantly reduce the frequency of composite outcome that includes stillbirth, or perinatal death, but it did reduce the risk of fetal overgrowth, shoulder dystocia, Cesarean delivery and hypertension (Landon M).
Fetal overgrowth is a complication of gestational diabetes and is associated with an increased risk of birth trauma in the form of brachial plexus injury or clavicular fracture, and of cesarean section to avoid such trauma.
Suggested thresholds of 140 mg % at 1 hour or 120 mg % at 2 hours to reduce the risk of macrosomia.
Management includes nutritional education, exercise, and blood glucose surveillance 4 times daily taken as fasting, or 1 or 2 hours postprandially.
Insulin therapy utilizes starting threshold fasting > 95 mg/dL; 1-hr levels ?140 mg/dL; 2-hr levels ?120 mg/dL.
Initial insulin starting dose: 0.7-1.0 units/kg daily in divided doses
Glyburide’s usual starting dose 2.5-20 mg daily in divided doses, and it likely crosses the placenta.
Metformin can be used in pregnancy.
Using metformin plus insulin to treat pre-existing type two or gestational diabetes does not reduce neonatal adverse outcome, but resulted in fewer large for gestational age infants (Boggess KA).
High-risk women with a diagnosis of diabetes at their first prenatal visit should be considered to have overt type 2 diabetes, rather than GDM.
Post-Partum screening for women with GDM should be performed 6 to 12 weeks after delivery.
Early screening should be performed in high risk women with a previous history of GDM, history of impaired glucose metabolism, or BMI ?30.
Retesting of the above women at 24-28 weeks if initial study is negative for GDM.
Screening tests include oral glucose tolerance tests.
Universal gestational diabetes screening is recommended at 24-28 weeks of gestation.
Criteria for diabetes: Fasting 92 mg/dL; 1-hr value 180 mg/dL; 2-hr value 153 mg/dL.
Initial management includes nutritional counseling, exercise, and blood glucose surveillance.
High-risk women with a diagnosis of diabetes at their first prenatal visit should be considered to have overt type 2 diabetes rather than gestational diabetes.
Lifelong screening and prevention of type 2 diabetes is advocated after gestational diabetes has been diagnosed.
Diagnostic threshold is fasting glycemic level of 126 mg/dL.
It is problematic to separate the role of fetalexposure to maternal hyperglycemia from maternaobesity and environmental exposures.
Infants borne of mothers with type II diabetes have a 6 fold increase of death within the first year and 11 times the risk of congenital malformations.
The risk of malformations increases with maternal fasting glucose, BMI, and early gestational age at diagnosis.
Malformations most commonly affect the cardiac, CNS and include transposition of the great arteries, septal defects, neural tube defects, and caudal regression syndrome.
Physical activity may lower risk of progression of gestational diabetes to type 2 DM (Bao W et al).
Maternal hyperglycemia promotes fetal hyperglycemia and stimulates fetal insulin secretion.
This suggests an excess of fetal adipose tissue.
Excessive fetal growth, macrosomia, is defined as an absolute birthweight of greater than 4000 to 4500 g.
Large for gestational age (LGA) is a birth rate greater than 90th percentile for gestational age.
Macrosomia is associated with asphyxia , perinatal death, shoulder dystocia with without birth injury, respiratory distress, and hypoglycemia, and hypocalcemia, hypomagnesemia, polycythemia, and hyperbilirubinemia can result.
CARDIA study of more than 2700 women suggests presence of gestational diabetes increased the risk of coronary artery calcification later in life, regardless of glucose control following their pregnancy.
Gestational diabetes is associated with increased risk of future endothelial dysfunction with advancing age.
Women with previous gestational diabetes had a twofold higher risk of coronary artery calcium if they maintained normal blood sugar levels, later developed prediabetes, or later were diagnosed with Type 2 diabetes many years after pregnancy compared to women without previous gestational diabetes who had normal blood sugar levels.
Diabetes and other health problems that develop during pregnancy serve as early harbingers of future chronic disease risk, particularly heart disease.
USPSTF recommends screening for gestational diabetes in asymptomatic pregnant persons at 24 weeks of gestation or after.
In randomized control trials it was found that treating women from 24 to 28 week gestation with gestational diabetes improves pregnancy outcomes: dietary advice, glucose monitoring, insulin therapy as required.
With such care there is a reduction in serious perinatal complications including death, shoulder dystocia, bone fracture, nerve palsy, fetal overgrowth, cesarean section, and hypertensive disorders,stillbirths, perinatal death and neonatal complications of hyper bilirubinemia, hypoglycemia, hyperinsulinemia, and birth trauma.
Reference guides for pregnant women should be followed including a minimum of 175 g of carbohydrates, 71 g of protein, and 28 g of fiber.
Limiting carbohydrates to 30 to 45% of total calories.
sugars and refined carbohydrates should be eliminated with ideal carbohydrate sources including fresh vegetables, some fruits, and whole grains.
Approximately 80% of women with gestational diabetes can reach the glycemic goals with diet and lifestyle lifestyle modifications alone.
In women with type one diabetes insulin regimens are effectice during pregnancy.
Continuous glucose monitoring and pregnant women with type one diabetes is efficacious and reduces the incidence of large for gestational age infants and lowers the incidence of hypoglycemia.
The use of oral hypoglycemic agents during pregnancy is controversial.
Metformin, a biguanide and glyburide has been used with good effect during pregnancy: metformin exposed children are larger than non-exposed children by several measures including weight and waste circumference.
Other sulfonylurea class of medication‘s are not recommended for use during pregnancy.
Women with diabetes are at increased risk for preeclampsia and low-dose aspirin is recommended after 12 weeks of gestation.
Maternal hyperglycemia during labor and delivery is associated with neonatal hypoglycemia and fetal distress.
Breast-feeding is encouraged in diabetic women as it facilitates postpartum weight loss and is likely associated with lower future risk of obesity and diabetes in offspring.
There is a rapid increase in insulin sensitivity following delivery of the placenta in women requiring insulin, and that the insulin dose should be reduced 1/3 to 1/2 of pre-delivery doses.
hybrid closed loop therapy significantly improves maternal glycemic control during pregnancy, complicated by type one diabetes (Tara TM).
