 Now used in the majority of intracoronary stenting procedures.
Now used in the majority of intracoronary stenting procedures.
Implanted in more than 500,000 patients in the US annually.
Used in 75-80% of angioplastic procedures.
The rationale for a drug eluting stent is that they impair the vascular healing process because of exposure of the metal in the stenr serves as a nidus for stent thrombosis.
Have controlled local release of antiproliferative agents.
The technology of drug eluting stents has evolved from early devices with heightened thrombotic risk, to third and fourth generation devices.
Newest stents are modified to retain the anti-restenosis properties while shortening their drug eluting properties to minimize the exposure of metal to platelets and fibrin:allowing for a shorter duration of dual antiplatelet therapy.
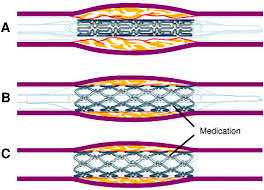
Three components: a metallic stent platform, a polymer coating, and an antiproliferative agent.
Stent platforms are available in stainless steel, cobalt or platinum chrome.
The use of metallic stents comes with limitations from permanently placing foreign material within the coronary artery, most notably, thrombus formation and vascula inflammation, both of which lead to new tissue growth, known as neointimal hyperplasia, resulting in some degree of vessel re-narrowing.
This process can lead to extensive neointimal tissue formation, and early restenosis or atherosclerosis, known as neoatherosclerosis.
Cobalt-chrome alloys have improved radial strength, increased radiographic popacity compared with stainless steel, allowing for a thinner struts with greater ability to deliver the stents.
Platforms with thinner struts may result in less arterial injury and reduce the risk of stenosis with lowered thrombogenicity.
Polymer coatings applied to the stent surface allow the ability to be drug carriers and permit drug release on a controlled basis.
The anti-proliferative agents that are used for the platforms of drug alluding stents are lipophilic molecules kwhich distribute to the arterial wall and exert immunosuppressive effects or anti-proliferative effects.
Safely reduces restenosis compared to bare metal stents.
Lower rate of repeat vascularization at 1 year when compared to bare metal stents.
The latest generation of drug eluding stent have low rates of in stent stenosis (6 to 8%) occurring primarily in the first year.
It is estimated that after the first year, drug eluding stands have an adverse ischemic event rate of 2% per year with no discernible plateau.
PCI interventions to treat stent restenosis now accounts for nearly 10% of all PCI’s performed in the US.
In stent restenosis is most commonly treated with repeat drug eluding stent placement, that is stent within his stent.
Randomized controlled studies suggest a 70-80% reduction in angiographic restenosis and 50-70% reduction in repeat target lesion or vessel revascularization compared to bare metal stents.
Reduce the need for repeat revascularization several times compared to bare metal stents.
Proven to be superior to bare metal stents in almost all types of lesions in native coronary arteries.
The reduction in angiographic and clinical restenosis with drug eluting stents has been reported to be between 50-70% depending on the complexity of coronary artery lesions treated.
Rates of lesion revascularization with sirolimus and paclitaxel eluting stents compared to bare metal stents reduced from 10-15% to approximately 4-5%, whereas rates of death, myocardial infarctions and stent thromboses were the same.
Meta-analysis of head to head trials revealed that sirolimus eluting stents are superior to paclitaxel eluting stents in relation to target lesion revascularization and stent thrombosis (Schomig).
With sirolimus not associated with an excess of subacute thromboses as compared to metal stents, when implanted with appropriate indications.
Everlolimus-eluting stents as compared to paclitaxel-eluting stebts associated with significantly reduced late lumen loss, and noninferior rates of asafety and efficacy (Stone GW).
Randomized trials have shown everolimus-eluting stent markedly reduces rates of death, myocardial infarction, restenosis, and stent thrombosis: everolimus eluting stents are safer and more effective than first-generation drug eluting stents.
Compared with everolimus-eluting metallic stents, everolimus-eluting bioresorbable vascular scaffolds have similar rates of repeat revascularization at 1 year follow-up with inferior midterm angiographic performance (Cassese S et al).
In a randomized trial of patients with multi vessel coronary artery disease comparing coronary artery bypass graft to PCI with everolimus-eluting stents: The rate of major cardiovascular adverse events was higher among those who had undergone PCI with Everlimus-eluting stents that among those who had undergone coronary artery bypass graft (Park S-J et al).
In a randomized study of 3687 patients to receive everolimus-eluting stents or paclitaxel-eluting stents: resulted in reduced rates of target lesion failure at 1 year, results consistent in al groups except thise with diabetes (Stone GW).
Early generation drug eluting stents releasing serolimus or paclitaxel from durable polymers reduce the need for repeat revascularization compared with bare metal stents.
Blood vessel healing is delayed with first generation drug eluting stents with evidence of chronic inflammation related,at least, in part to the persistence of durable polymer components in patients with acute STEMI.
The meta-analysis by the Drug Eluting Stent in Primary Angioplasty (DESERT) Cooperation demonstrated that among patients with STEMI undergoing primary primary percutaneous coronary interventio, sirolimus eluting and paclitaxel eluting stents compared with bare metal stents are associated with with reduction in target vessel revascularization at long-term follow-up, without differences in cumulative mortality, reinfarction or stent thrombosis.
In the above study the incidence of very late reinfarction and stent thrombosis was increased with drug eluting stents (De Luca G et al).
Recent studies suggesting that long term use of such stents may increase coronary artery thrombosis and death, as a result of stent sites not healing thus increasing risk of blood clots.
Late restenosis, may occur particularly in complex lesions or in those with high risk of complications such as diabetes and those with multiple vessel disease.
Proposed that late clinical stenosis may be due to delayed arterial healing after implantation of drug eluting stents (Nakazawa G).
Associated with rates of late stent stenosis of 8.9-18.9% in complex lesions.
Randomized comparative studies of drug eluting stents vs bare metal stents demonstrates a low frequency of approximately 0.5%, relative increase in incidence of very late stent thrombosis, that becomes evident after 9-12 months and extends to 3-4 years.
Relative incremental risk for late stenosis with drug eluting stents is 0.2% per year through 3 years.
Late stent thrombosis does not result in increased risk of death or myocardial infarction, but raises concerns about long-term safety of drug eluting stents.
With late stent thrombosis evidence of incomplete endothelialization, delayed arterial healing and vessel remodeling occur due to chronic inflammation.
The presence of durable polymer material on the stent surface. Following drug release completion was felt to be a potential trigger for chronic inflammation leading to very late stent thrombosis.
In a study of biodegradable polymer biolimus eluting stents vs. durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS) revealed non-inferiority, and by reducing the risk of cardiac events associated with very late stent thrombosis may improve long-term clinical outcomes for up to four years (Stefani GG et al).
Present recommendation for the use of clopidogrel is for at least 3 months for sirolimus coated stents and 6 months for paclitaxel coated stents after implantation.
Present guidelines recommend clopidogrel at a dose of 75 mg daily for at least 12 months after implantation of drug eluting stents, if patients are not at high risk for bleeding (King SBIII).
In a dual antiplatelet study (ASA vs. ASA + clopidogrel) of greater than 12 months in patients with drug eluting stents dual treatment was not significantly more effective than aspirin monotherapy in reducing rate of myocardial infarction or death from cardiac causes (Park Seung-Jung).
Premature discontinuance of antiplatelet treatment associated with stent thrombosis.
A pooled analysis of 4 randomized trials comparing sirolimus eluting stents and bare metal stents in 1748 patients with a 4 year follow-up did not find evidence of a significantly higher rate of death, myocardial infarction or stent thrombosis in the sirolimus group. Survival rate at four years was 93.3% in the sirolimus stent group and 94.6% in the bare metal group.
Approved for patients with previous untreated coronary artery lesions of less than 30 mm in length and a reference vessel diameter of 2.5-3.75 mm.
The use of such stents have been expanded to more complicated lesions and in acute settings.
