 The complement system is composed of greater than 20 proteins that act in concert to generate products that have both immunoprotective and proinflammatory properties.
The complement system is composed of greater than 20 proteins that act in concert to generate products that have both immunoprotective and proinflammatory properties.
A key component of innate immunity and the regulator of tissue homeostasis.
Found in greatest concentration in the plasma.
More than 50 soluble and membrane-bound proteins form the complement system, providing innate defense against microbes and mediating inflammatory responses.
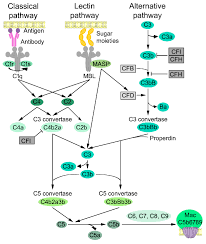
Activated via three different pathways: classical, alternative, and lectin.
Importantly, the alternative pathway of complement serves as an amplification loop for the lectin and classical pathways, accounting for roughly 80% of complement activation products.
The complement system is activated by immune complexes as in the classic pathway, or microbial mannose as in the lectin pathway.
The classic and lectin pathways are augmented by the alternative pathway, and which complement factors B and D promote the generation of alternative C5 convertase, C3bBbC3b.
The alternative pathway, view the action of complement factor P, perpetually promotes the generation of alternative C3b converttase and C3b convertase, resulting in a brisk activation of the complement system for host defense.
The alternative complement system is regulated by a multitude of regulators that include plasma proteins and membrane proteins.
On the surface of blood cells and other cells, glycophosphatidylinositol anchored CD55 and CD59 are involved in the regulation of the alternative complement pathway.
In PNH, somatic mutation of the PIGA gene in a hematopoetic stem cell causes deficiency of a glycophosphatidylinositol in its progeny cells: as a result, excessive activation of the complement system occurs on the surface of the affected blood cells, resulting in intravascular hemolysis and thrombosis.
All activating pathways converge to complement component 3-mediated amplification loop by pathway-specific C3 convertases.
The classical pathway is activated by an antigen-antibody immune complex or C-reactive protein, and the lectin pathway is activated directly by mannose-containing bacterial surfaces.
The classical pathway is mainly activated by antibody-antigen complexes recognized via complement component C1q.
The liver is the major site for the synthesis of most complement molecules.
Besides hepatocytes, other cell types, including monocytes, macrophages, endothelial cells, fibroblasts, and adipocytes, can be local sources of complement proteins such as C1q, C3, and C529.
Among them, both activating molecules (C3, C4, C9, and factor B) and negative regulators (C1 inhibitor and C4BP) are up-regulated during an acute-phase reaction, which underlines the relevance of activating balanced complement-mediated responses.
Among antibody isotypes, IgM is the most effective in activating complement.
Activation of complement with the four subclasses of IgGs varies as a function of steric hindrance by the Fab arms in the approach of C1q to the IgG CH2 sites (IgG3>IgG1>IgG2>IgG4).
The system functions by innate and adaptive immunity for defense of microbes.
Activation results formation of a number of components that increase vasculature permeability, chemotaxis and opsonization.
Present in inactive forms in plasma with numeration of C1-C9.
Many complement proteins are activated to become proteolytic enzymes that degrade therapy complement proteins forming a cascade effect.
Plays a role in acute inflammatory response associated with ischemia and reperfusion injury.
C3a and C5a which are anaphylatoxins, and C5b-9 membrane attack complex formed during complement activation promote tissue injury by increasing vascular permeability, activates endothelium, activates hemolysis, induces cell lysis, and induces apoptosis.
