The
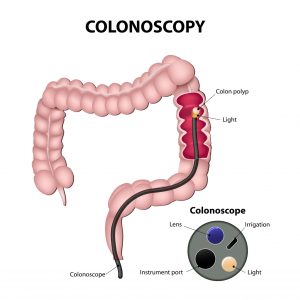 About 19 million procedures performed each year in the U.S.
About 19 million procedures performed each year in the U.S.
That’s 52,000 per calendar day.
Colonoscopy performance is highly operator dependent.
On a per-lesion basis, colonoscopists with the worst performance miss more than 90% of pre-cancerous colorectal lesions.
On a per-patient basis,: colonoscopists with the worst performance, in terms of adenoma detection rate, diagnose more than half of patients who have a pre-cancerous colorectal lesions as normal.
The adenoma detection rate refers to the fraction of patient’s age 50 years and older undergoing first time screening colonoscopy who have had at least one conventional adenoma detected by a colonoscopist.
The Adenoma detection rate (ADR) is considered the most important measure of colonoscopy quality:minimum threshold recommended for acceptable performance is 30% for men, 20% for women, or 25% for a patient population that includes men and women.
Bleeding after polypectomy occurs in 1% of persons.
Endoscopist unable to reach the cecum in up to 15% of patients.
The main diagnostic tool for diagnosing diverticular disease.
Asymptomatic diverticular disease is a frequent incidental finding on screening colonoscopy.
Not performed during acute diverticulitis because of the possible complication of perforation.
1 in every 1000 colonoscopies complicated by perforation, 3 are complicated by major hemorrhage and mortality rate is 1 to 3 in 10,000 procedures.
With a perforation rate of bout 0.8%, 113,6000 perforations can be expected annually.
Respiratory depression from sedation occurs in one in 200 patients.
Perforation rate is at least 25 times greater than that of double-contrast barium enema.
Serious complications-perforation and problems with conscious sedation occur in less than 0.2% of examinations by gastroenterologists.
Less commonly complications include pneumothorax, mesenteric tears, volvulus, sepsis, retroperitoneal abscess and rupture of the spleen.
The risk of complications is increased in older adults and include perforation, G.I. bleeding, and cardiovascular risks associated with anesthesia.
Sensitivity for cancer detection is approximately 95%.
110,000 new colon cancer diagnoses per year.
Colonoscopy reduces the risk of death from colorectal cancer by detecting tumors at an earlier, more treatable stage through removal of precancerous adenomas.
Colonoscopy is associated with a 40 to 69% decrease in the incidence of colorectal cancer and a 29 to 88% decrease in the risk of death from this cancer.
Approximately 25% of colonoscopies are for adenoma surveillance.
Failure to detect adenomas during colonoscopy may increase subsequent risk of cancer.
Currently recommended adenoma detection rates of 20% or higher for females and 30% or higher for male patients as an indicator of colonoscopy quality.
Study of 42 interval colon cancers identified over five years indicated an association between colonoscopy related adenoma detection rate of less than 20% versus greater than 20% and the risk of interval cancer (Kaminski MF et al).
Adenoma detection rate is inversely associated with the risks interval colorectal cancer, advanced stage interval cancer, and fatal interval cancer (Corley DA et al).l
Sensitivity to detect adenomas larger than 1 cm in about 90% and sensitivity for detection of adenomas smaller than 1 cm may be as low as 75%.
Colonoscopy has been estimated to identify only 75% of small pre-cancerous lesions.
Best age for a single colonoscopy to save most life years from death due to colorectal cancer would be between 65 and 70 years.
In patients with no findings on screening colonoscopy may undergo repeat screening colonoscopy 10 years later.
Regular colonoscopy with polypectomy, when indicated, achieve a reduction on the incidence of colorectal cancer incidence of approximately 75-90% compared to reference populations in the National Polyp Study.
Several studies have been done with baseline colonoscopy and repeated exams within 2-5 years to detect interval neoplasia found 0.3% to 0.9% of patients have interval cancers within only a few years after colonoscopy and polypectomy.
Repeat study within 10 years after negative screening study may be overuse.
Explanations that lesions can appear in the short amount of time explained by new de novo lesions, rapidly growing lesions, missed lesions, or incompletely removed lesions that can account for these interval cancers.
Chemoendoscopy with special stains or narrow band imaging or fluorescence endoscopy may help visualize and demarcate flat lesions at colonoscopy.
The gold standard for colorectal cancer surveillance in prevention trials.
The most accurate test for early detection and prevention of colorectal cancer, markedly reducing the risk of colorectal cancer and death.
Patients with no abnormality on a previous colonoscopy have a markedly reduced risk of colorectal cancer.
Use of screening colonoscopy is associated with a reduction in the incidence of colorectal cancer of 67% and a reduction in the rate of death of 65% (Kahi CJ et al).
Significant variation in rates of detection of cancer among experienced gastroenterologists and is related the withdrawal times.
Longer withdrawal times correlate with increased rates of neoplasia detection.
6 minutes is the recommended estimated time that is necessary for adequate inspection of normal colons, and rates of detection of neoplasms is greater for endoscopists with a longer mean procedure withdrawal time than for those who have shorter withdrawal times.
Inverse correlation between mean withdrawal time and mean polyp size detected.
The American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG) call for adenoma detection rates of at least 25% in men and 15% or more in women age 50 years or older on colonoscopy screenings.
The reach-the-cecum rate should be about 90% or greater in all cases and at least 95% in screening colonoscopies.
Perforation rates higher than 1 in 500 overall or 1 in 1000 in screening colonoscopies would be excessive and would suggest quality impairment or quality deficiency.
Use of anesthesia associated with somewhat higher risk of complications, especially, aspiration pneumonia (Cooper GS et al).
The degree of bowel cleansing is critical in colonoscopy and bowel preparation is inadequate in up to 30% of cases and decreases diagnostic accuracy, prolongs the time for the procedure, decreases surveillance intervals, increased costs and potentially results in procedure related complications.
Bowel preps should clear the colon, provide maximum visualization of the mucosa, preserve the gross and microscopic integrity of the colon, be easy to administer, be safe and well tolerated.
An inadequate preparation may be defined by the presence of excess solid or semi solid stool that could not be irrigated and suctioned, therefore, certainly lesions such as polyps or cancers may be missed.
There are bowel preparation scores which should be used to evaluate how the prep was described and if it was defined using an objective system.
An inadequate bowel preparation can lead to an increased risk of adverse events, as well as decreased rate of detection for adenoma.
An inadequate preparation of the bowel is the most important risk factor for an inadequate preparation in the future.
Risk factors for an inadequate preparation include: male sex, history of opioid use, history of constipation, the presence of diabetes, cirrhosis, obesity, Parkinson’s disease, and a poor health literacy.
Colonoscopy preparations are divided into two categories polyethylene glycol-based and hyper osmotic agents.
There are two primary protocols for the administration of a bowel preparation: single dose and split dose.
In the sigle dose prep the patient takes the entire volume, usually 2-4 L, the night before the procedure or the day of the procedure.
In the split-does prep, half of the prep is given the evening before the procedure, followed by the second dose given approximally five hours before the procedure.
It is important to complete the second half of the prep no later than two hours before the procedure to avoid potential delays in receiving anesthesia.
Split dosing has become the preferred modality for bowel prep as multiple studies have shown it is a good or excellent preparation in about 85% of patients versus 63% of those who did not receive a split dose
Split dosing increases both the adenoma detection rate and polyp detection rate.
Retrospective studies suggest aspirin can be safely continued during colonoscopy with polypectomy without concern for a significant increase in bleeding.
NSAIDs, which reversibly block cyclooxygenase, can be used safely prior to colonoscopy procedures.
Continuing thienpyridine agents during polypectomy is estimated the risk of clinically important post polypectomy bleeding at 0.9 to 2.1%.
The US multi Society task force on colorectal cancer recommends:
Patients with one to two tubular adenomas less than 10 mm can extend surveillance colonoscopy to 7 to 10 years rather than 5 to 10 years.
Patients with 3 to 4 tubular adenomas less than 10 mm can extend follow up of surveillance to 3 to 5 years rather than three years.
Patients with 11 adenomas or greater should have repeated surveillance in one year rather than less than three years.
Patients with advanced adenomas greater than 10 mm, tubovillous or villous features for high-grade dysplasia should have repeat colonoscopy in three years.
A cohort study found that in a population without a family history of colorectal carcinoma, the 10 year interval between colonoscopy screenings for individuals with the first colonoscopy with findings of negative for CRC could potentially be extended to 15 years (Laing Q).
