
The choroid plexus is a plexus of cells that arises from the tela choroidea in each of the ventricles of the brain.
The choroid plexus produces and secretes most of the cerebrospinal fluid (CSF) of the central nervous system.
The CP consists of modified ependymal cells surrounding a core of capillaries and loose connective tissue.
There are multiple cilia on the ependymal cells move to circulate the cerebrospinal fluid.
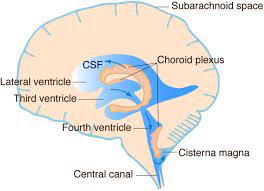
There is a choroid plexus in each of the four ventricles.
The choroid plexus (CP) of the blood-cerebrospinal fluid barrier (BCSFB) impacts CSF homeostasis, brain diseases, and neuromedical translation. CP executes neuroendocrine, excretory, and neuroimmune actions.
There is no choroid plexus in the anterior horn.
In the third ventricle there is a small amount in the roof that is continuous with that in the body, via the interventricular foramina, the channels that connect the lateral ventricles with the third ventricle.
A choroid plexus is part of the roof of the fourth ventricle.
The epithelium of the choroid plexus is continuous with the ependymal cell layer that lines the ventricular system.
Unlike the ependyma, the choroid plexus epithelial layer has tight junctions, between the cells on the side facing the ventricle.
These tight junctions prevent the majority of substances from crossing the cell layer into the cerebrospinal fluid (CSF); acts as a blood–CSF barrier.
The choroid plexus surface area is increased due to its folding into many villi around each capillary, creating frond-like processes that project into the ventricles: there is a brush border of microvilli, that greatly increases the surface area of the choroid plexus, as well.
As plasma is filtered from the blood through the choroid epithelial cells CSF is formed.
Choroid plexus epithelial cells, then actively transport sodium ions into the ventricles and water follows the resulting osmotic gradient.
The choroid plexus consists of many capillaries, separated from the ventricles by choroid epithelial cells.
Fluid filters through these cells from blood to become cerebrospinal fluid.
There is active transport of substances into, and out of, the CSF as it is made.
The choroid plexus regulates the production and composition of CSF, that provides the protective buoyancy for the brain.
BCSFB manages brain-spinal cord fluid environments, giving rise to a wide pathophysiology spectrum.
Choroidal plexus phenomena include integration of circadian clock signals, immune interaction with gut microbiotica, and expression of receptors to taste CSF composition.
Recent studies estimate that approximately 80% of the CSF is secreted by the CPE, with the remaining 20% coming from the brain interstitial fluid.
CSF is replaced three to four times per day.
With the rapid secretion of CSF, if there is an associated blockage in the circulatory pathway, it can result in a
ventricular dilation and even increased intracranial pressure, eventually leading to hydrocephalus (obstructive hydrocephalus).
Communicating hydrocephalus, on the other hand, is relatively uncommon due to an obstruction of CSF circulation, but rather an obstruction of CSF absorption.
Either intrinsic genetic factors or external environmental factors that substantially promote the secretion of CSF from the choroid plexus or disrupt the reabsorption of CSF.
This leads to the accumulation of CSF in the ventricles and the development of hydrocephalus.
It is suspected that changes in osmotic pressure between CPE and capillaries can also lead to abnormal accumulation of CSF, which in turn leads to hydrocephalus.
This is the relationship between the choroid plexus and hydrocephalus, and the theory behind choroid plexus cautery.
CSF is the medium for the glymphatic filtration system that facilitates the removal of metabolic waste from the brain, and the exchange of biomolecules and foreign substances into and out of the brain.
The choroid plexus has a very important role in helping to maintain the extracellular environment required by the brain to function optimally.
The choroid plexus is also a major source of transferrin secretion that plays a part in iron homeostasis in the brain.
Similar to the blood–brain barrier, the blood–CSF barrier functions to prevent the passage of most blood-borne substances into the brain, while selectively permitting the passage of specific substances into the brain and facilitating the removal of brain metabolites and metabolic products into the blood.
The blood brain barrier and blood CSF barrier facilitate the transport of different substances into the brain.
The have distinctive structural characteristics of each of the two barrier systems.
The blood–cerebrospinal fluid barrier modulates the entry of leukocytes from the blood to the central nervous system.
The choroid plexus cells secrete cytokines that recruit monocyte-derived macrophages to the brain: implicating normal brain homeostasis and neuroinflammatory processes.
During fetal development, some choroid plexus cysts may form, with a prevalence of ~1%.
Choroid plexus cysts are usually an isolated finding, and typically disappear later during pregnancy, and are usually harmless.
Choroid plexus cysts have no effect on infant and early childhood development.
Cysts confers a 1% risk of fetal aneuploidy.
44-50% of Edwards syndrome (trisomy 18) cases will present with choroid plexus cysts, as well 1.4% of Down syndrome (trisomy 21) cases.
About 75% of abnormal karyotypes associated with choroid plexus cysts are trisomy 18, while the remainder are trisomy 21.
