Cervical screening is the process of detecting and removing abnormal tissue or cells in the cervix before cervical cancer develops.
The objective of cervical screening is to detect and treat cervical neoplasia early on, with the aim of secondary prevention of cervical cancer.
Over a lifetime, cervical cancer develops in up to 5% of an unscreened population, and effective screening and treatment of cervical pre-cancers can reduce the lifetime risk to less than 0.5%.
Approximately 100,000 people are treated for cervical pre-cancer each year in the US to prevent cervical cancer.
up to 20% of individuals have prior abnormal results, prior pre-cancer, or cancer, or immunosuppression, and require a screening at 1 to 3 year intervals that are considered to be under surveillance or undergoing high risk screening.
Pre-cancers are abnormal cervical cells that progress to cancer, unless treated and include high grade squamous intraepithelial lesions (HSIL) , cervical intraepithelial, neoplasia, grade 3 ( CIN3), and adenocarcinoma in situ.
14,000 people are diagnosed with cervical cancer and more than 4000 die from cervical cancer each year, mostly due to lack of screening.
Currently approximately half of cervical cancers occur in people with inadequate screening and up to 25% of individuals in the US are underscreened.
Several screening methods for cervical cancer are the Pap test or conventional cytology, liquid-based cytology, the HPV DNA testing and the visual inspection with acetic acid.
Virtually all cervical cancers contain, at least one of 13 carcinogenic HPV genotypes.
Carcinogenic HPV genotypes are linked in a single branch of the alpha genus: alpha 9, -7, -5, and -6 species contain HPV genotypes that are carcinogenic.
HPV 16 is the most carcinogenic associated with more than 60% of cervical squamous cancers, and adenocarcinomas and oropharyngeal and other anorectal cancers.
Patients with a cervix should be screened with HPV testing, and if HPV positive, genotyping and cytology testing, should be performed to assist the risk of cervical pre-cancer and determine the need for colposcopy or treatment.
The risk of pre-cancer can be estimated by identifying the HPV genotype and using cytology and P 16/KI 67 dual staining to understand whether the HPV infection is replicating or abortive and transforming and more likely pre-cancerous.
Pap test and liquid-based cytology have been effective in diminishing incidence and mortality rates of cervical cancer in developed countries but not in developing countries.
Programs of repeated cytology, screening, cool, colposcopy guided biopsiess, and excision of pre-cancerous changes of the cervix have reduced population level, cervical cancer incidence and mortality by 60 to 80%.
The sensitivity of cytology for detecting pre-cancer is 50 to 70% compared with more than 90% for HPV testing.
97% of pre-cancers are HPV positive so performing concurrent, cytology and HPV testing provides limited additional information compared with HPV testing alone.
Prospective screening methods that can be used in low-resource areas in the developing countries are the HPV DNA testing and the visual inspection.
The risk of cervical cancer begins to increase around 30 years and remains elevated for the remainder of the lifespan.
Screening is recommended at least every five years for individuals age 25 through 65 years who have a cervix.
The USPSTF recommends screening average risk individuals with cytology alone at ages 21 through 29 years and HPV testing alone, HPV testing with cytology, or cytology alone at ages 30 to 65 years.
ACS guidelines recommend HPV testing alone at five year intervals from age 25 to 65 years.
Estimated 14,480 women will be diagnosed with cervical carcinoma in 2o22 with 4300 expected deaths.
Approximately 100,000 people are treated for cervical pre-cancer each year in the US to prevent cervical cancer.
It is estimated that 80% of cervical cancer mortality can be reduced by screening: yet, there has been a 14 year decline in screening in the US.
Currently, approximately half of cervical cancers occur in people with inadequate screening, and up to 25% of individuals in the US are underscreened.
Approximately 20% of the US population requires more frequent screening due to prior cervical cancer screening abnormalities or immunosuppression.
Programs of repeated, cytology screening, colposcopically guided biopsies, and excision of pre-cancerous changes of the cervix have reduced population levels cervical cancer incidence, and mortality by 60-80%.
Should be started approximately 3 years after onset of sexual activity, at least by age 21.
No evidence of cervical cancer screening in adolescents has decreased incidence of cervical cancer.
Not required for women with prior hysterectomy for benign lesions.
The US preventive Services Task Force calls for screening to begin no earlier then age 21 years and with an interval of three years between routine Pap tests for women age 22-30 years.
U.S. Preventive Services Task Force recommends screening of women for cervical cancer with basic cytology starting at age 21 years, and consider adding high-risk human papillomavirus (hrHPV) testing alone or with cytology for women aged 30 years and older.
The US preventive Services Task Force recommendations on screening for cervical cytology is every 3 year screening of Pap testing alone, or an every 5 year screening with HPV testing alone for women ages 30-65.
The USPSTF recommends against screening for cervical cancer in women older than 65 years who have had an adequate prior screening and not otherwise at high-risk for cervical cancer.
The USPSTF recommends against screening for cervical cancer in women younger than 21 years.
The USPSTF recommends against screening for cervical cancer in women who have had a hysterectomy with removal of the cervix and do not have a history of high-grade pre-cancerous lesions or cervical cancer.
Screening for women over the age of 65 is not recommended, but should have documentation of at least three consecutive negative cytology results or two negative consecutive HPV test results within the past 10 years and no abnormal results in the past 10 years, no history of cervical pre-cancer in the past 25 years, no history of cervical cancer, and no immunosuppression.
Because of cytological and histological variability, including information related to HPV infection increases the accuracy of prevention strategies.
The risk of pre-cancer can be accurately estimated by identifying the HPV genotype and using morphological and biological test, such as cytology and P 16/KI 67 dual stain, to understand whether the HPV infection is replicating or abortive and transforming.
The squamocolumnar junction of the cervix is particularly susceptible to HPV carcinogenesis; changes in cells that are precursors to cervical cancer typically develop in this area.
American cancer society recommendations for cervical cancer screening:
Begin screening at age 25 years regardless of sexual history or HPV vaccination status
If primary HPV testing is not available use cotesting HPV plus cytology every five years or every three years with cytology only.
Discontinue screening at age 65 with no history of cervical intraepithelial neoplasia grade 2 or more severe diagnosis in the last 25 years and adequate negative prior screening in the last 10 years.
hrHPV and hrHPV plus cytology were associated with higher rates of false-positives and colposcopy compared with cytology alone.
hrHPV testing yielded higher rates of positive cervical intraepithelial neoplasia, compared with cytology alone as initial screening.
In women who have never been sexually active has little value.
Immunosuppressed or immunocompromised adolescence should undergo cervical screening age 21.
Should be done in women who have had vaccination against HPV 16 and 18, because other high risk subtypes of HPV are oncogenic.
After 3 consecutive years of normal tests and with no dysplasia within the past 5 years less frequent screening may be done, but should be performed every 3 years.
99% of cervical cancers contain the genes of HPV’s, most commonly 16, 18, 3, 33,35, 39, 45, 51, 52, 56, 58, 59 and 68.
Carcinogenic HPV belong to the alphapapillomavirus genus.
HPV 16 most common carcinogenic type, accounting for half of cervical cancer cases.
HPV 18 implicated in endocervical adenocarcinoma, accounting for 15% of cervical cancers.
Combinations of HPV DNA testing, traditional PAP smears and liquid based cytology have improved sensitivity and specificity of cervical cancer screening.
HPV testing accepted associated adjunct to cytologic testing for women over the age of 30 years.
Primary screening for human papilloma virus (HPV) using a DNA test can be considered as an alternative to current US cytology-based cervical cancer screening strategies.
Cervical screening is recommended for women between ages 21–65, regardless of age at sexual initiation or other high-risk behaviors.
For healthy women aged 21–29 who have never had an abnormal Pap smear, cervical cancer screening with cervical cytology (Pap smear) should occur every 3 years, regardless of HPV vaccination status.
The preferred screening for women aged 30–65 is includes a combination of cervical cytology screening and HPV testing, every 5 years.
It Is also acceptable to screen this age group with a Pap smear alone every 3 years.
In women over the age of 65, screening for cervical cancer may be discontinued in the absence of abnormal screening results within the prior 10 years and no history of high-grade lesions.
All cervical cancer screening is discontinued for individuals aged >65 years with no history of cervical intraepithelial neoplasia grade 2 or more severe disease within the previous 25 years or with documented adequate negative prior screening in the previous 10 years.
The greatest impact on cervical cancer reduction appears to result from screening women aged 30 to 39 years.
Studies of the accuracy of conventional cytology report:
sensitivity 50%
specificity 94%
Liquid-based monolayer technique based on placing the sample into a vial containing a liquid medium that preserves the cells have been increasingly used.
Overall, as compared with conventional cytology analysis, liquid-based cytologic analysis is similar or higheer sensitivity for the detection of cervical intraepithelial neoplasia (CIN) of grade 2 or higher and grade 3 or higher, a similar lower specificity and positive predictive value, and a lower proportion of unsatisfactory slides.
Studies suggest that screening with liquid-based cytology reduces the incidence of and the mortality associated with cervical cancer.
Two of the types are Sure-Path (TriPath Imaging) and Thin-Prep (Cytyc Corp) are used.
The liquid sample is suitable for high-risk HPV testing and may reduce unsatisfactory specimens from 4.1% to 2.6%.
Studies of the accuracy of liquid based monolayer cytology report:
sensitivity 61%-66%,
specificity 82%-to 91%
Studies of the accuracy of HPV testing report:
sensitivity 88% to 91% for detecting CIN 3 or higher to 97% for detecting CIN2+
specificity 73% to 79% for detecting CIN 3 or higher to 93% for detecting CIN2+
By adding the more sensitive HPV test, the specificity may decline.
Declining specificity results in increased numbers of false positive tests and, an increased risk for colposcopy, an invasive procedure and unnecessary treatment.
HPV testing appears as sensitive as immediate colposcopy while reducing the number of colposcopies needed.
The act of performing a Pap smear produces an inflammatory cytokine response, which may initiate immunologic clearance of HPV, therefore reducing the risk of cervical cancer
Women with even a single Pap smear in their history had a lower incidence of cancer.
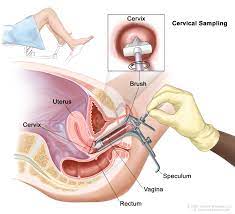
A Pap smear, collects cells using a spatula then smeared onto a slide for examination under a microscope.
In a liquid-based cytology test, a sample of cells is taken using a small brush and put it into a container of liquid, and analysed for abnormalities.
Cervical cells to be tested for HPV are collected in a similar way.
Abnormal cells can be removed or destroyed by one of several different procedure:
Laser ablation
Cryotherapy
Loop electrical excision
Cervical conization
Laser ablation and cryotherapy treat just part of the cervix that contains abnormal cells.
These procedures allow normal cells to grow back in their place.
The loop electrical excision procedure, cervical conization and hysterectomy remove the whole area containing the cells that could become pre-cancerous or develop into cervical cancer.
With HPV testing, there is 50 percent reduction in the number of deaths from cervical cancer compared to unscreened women.
HPV testing has the fewest false negatives.
Visual inspection with acetic acid has shown to have in several studies a low specificity compared to cytology and a high rate of false positives.
HPV screening is highly sensitive, but specificity depends on subsequent evaluation strategies and screening frequencies.
Evidence now supports primary HPV testing alone for screening.
Recommendations are presented for cervical cancer screening of individuals at average risk.
The researchers recommend initiation of cervical cancer screening at age 25 years and continuing primary human papillomavirus (HPV) testing every five years through age 65 years.
If primary HPV testing is not available, cotesting, HPV testing in combination with cytology, should be performed every five years for individuals aged 25 to 65 years or cytology alone should be performed every three years.
Compared with cytology-only screening performed at the same interval, primary HPV screening offers better reassurance of low cancer risk.
Primary HPV screening can be considered as an alternative to cytology alone, cotesting, and other current US cytology-based cervical cancer screening approaches.
Specificity of HPV screening is higher in women 35 years of age or older than younger women because the infection is transient and the peak infection occurs in women in their early twenties.
HPV DNA testing is not recommended in women younger than 21 years.
The five-year cumulative incidence of HSIL+ and CIN-2 positive analyses was similar in HIV-infected women and HIV uninfected women who were cytologically normal and oncogenic HPV negative at an enrollment study (Keller MJ et al).
Screening can lead to unnecessary testing and treatment for lesions that would spontaneously regress.
A single Pap test is limited sensitivity for the detection of cervical cancer and it’s immediate precursors.
To compensate for it’s limited sensitivity repeat screening at short intervals and a low cytologic threshold for additional follow-up is required.
Expenditures in the US total $6 billion annually more than 50 million screening tests.
2015 recommendations for cervical cancer screening: average risk women under age 21 should not be screened for cervical cancer.
Once average risk women reach the age 21 they should be screened once every three.
Average risk women should not be screen with cytology more frequently than every three years.
Average risk women age 30 years or older who prefers screening less often than every three years should undergo cytology and HPV testing once every five years.
Average risk women younger than 30 years should not have HPV testing performed.
Average risk women older than 65 years should not continue being screened for cervical cancer if they had three consecutive negative cytology results or two consecutive negative cytology with HPV test results within a 10 year period, the most recent test performed within five years.
Average risk women of any age should not be screened for cervical cancer if they had a hysterectomy with removal of the cervix.
In a study of screened patients-more than 1.5 million individuals aged 25-65 years, 90% test were normal and 0.75% were severely abnormal:The remainder were minimally abnormal category that includes human papilloma virus positive test with concurrent normal cytologic interpretation, atypical squamous cells of undetermined significance, and low-grade squamous into epithelial lesion.
Cytology screening is not as effective in young women who were vaccinated with HPV vaccine because it is more likely than HPV testing to pick up minor abnormal cell changes unrelated to HPV type 16 and 18, which caused nearly all cervical cancers.
As HPV vaccination rates rise, false positive findings from Papanicolaou tests are expected to rise significantly, leading to unnecessary extra testing or treatment, which can permanently alter the cervix and cause serious complications for future pregnancies among other problems.
The addition of cytology to HPV testing provides minimal benefit well leading to more false positive results.
