 Tumor suppressor genes inherited in an autosomal dominant fashion.
Tumor suppressor genes inherited in an autosomal dominant fashion.
Loss-of function genes.
In the general population, approximately 1 in 400 people have a BRCA1/2 mutation.
The prevalence of BRCA is 10 times higher in the Ashkenazi Jewish population.
Both BRCA1 and BRCA2 tumor suppressor genes are involved in DNA repair by homologous recombination.
BRCA1 and BRCA2 are important components of the homologous recombination repair pathway and can trigger accumulation of DNA damage-mediated double stranded breaks.
Loss of BRCA function causes homologous recombination repaired deficiency, resulting in dependence on the less efficient mechanisms of DNA repair and leads to accumulation of unrepaired DNA genomic instability and cell death.
They are involved in different types of DNA damage, such as single, strand, or double strand breaks, based damage bulky – adducts, cross links, or replication lesions.
A single copy of the BRCA1or BRCA2 is sufficient to repair DNA brakes, and asecond loss or double hit is needed to impair this pathway.
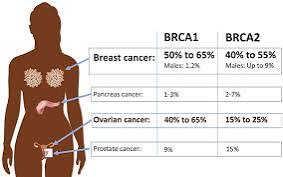
BRCA1 and BRCA2 variant are highly penetrant for breast and ovarian cancer and are associated with other cancers such as pancreas and prostate, serous uterine cancer.
BRCA1/2 mutations occur in 5-10% of breast cancer cases and 15% of ovarian cancers.
BRCA1/2 gene mutations increase breast cancer risk by 45-65% by age 70 years.
Risk of ovarian, fallopian tube, or peritoneal cancer increases to 39% for BRCA 1 mutations and 10-17% for BRCA 2 mutations.
Cells with a deficiency in homologous recombination repair, such as those with a BRCA mutation, are sensitive to PARP inhibition through multiple mechanisms.
Cells with alterations in homologous recombination pathway genes are unable to repair DNA double strand breaks by homologous recombination.
BRCA genes code for proteins that are involved in homologous recombination repair of the DNA double-strand breaks.
More than 1800 variants of been reported for BRCA1 and 2000 variants for a BRCA2.
Among patients with BRCA mutation the cumulative risk of developing breast cancer by the age of 80 is 67%-72% in BRCA1 carriers and 66%-69% in BRCA2 carriers.
Breast cancer peak incidence occurs slightly earlier for BRCA1 mutation is at ages 41-50 years compared with ages 51-60 years in BRCA2 carriers.
The degree of risk, that is, penetrance, of developing breast or ovarian cancer depends on the location and type of mutation, cooccurring single nucleotide polymorphisms, ethnicity, and environmental factors. .
Ovarian cancer asociated with 10-15% of cases with germline mutations in BRCA1 and BRCA2.
BRCA1 and BRCA2 dysfunction, BRCAness, may be seen in up to 20% of unselected ovarian cancers (Hennessy B).
BRCA1 methylation decreases BRCA1 expression in 5-31% of sporadic ovrian cancers (Baldwin RL).
Amplification of EMSY, a BRCA2 inhibitor, seen in 17% of sporadic ovarian cancers (Hughes-Davies L).
Increased risk for prostate cancer, male breast cancer and pancreas cancer.
BRCA1 and BRCA2 variant cancers in men associated with poor clinical outcomes.
BRCA associated with about 5% of pancreatic cancers.
Epigenetic and genetic mechanisms can create BRCAness phenotype in about one third of cases of ovarian cancers .
In BRCA mutation carriers MRI can detect early breast cancers with 75-94% of screen detected invasive tumors being 2 cm or smaller at diagnosis and 75-83% being negative for axillary node metastases (Kriege M, Kuhl, CK).
BRCA1 and BRCA2 are the most common of high-risk mutations and confer a greater than 11-fold increase in breast cancer risk.
BRCA1 and BRACA2 associated with a lifetime risk of breast cancer of 66-76% by age 80 years.
A substantial gain in life expectancy of 2.9-5.3 years results from risk reducing mastectomy and gains of 0.3-1.7 years can be achieved with risk reducing salpingo-oophorectomy, depending upon the level of cancer risk and in young women with BRCA1 mutations (Schrag D).
It is estimated that ovarian cancer risks range from 36-63% for BRCA1 mutation carriers and 10%-27% for BRCA2 mutation carriers.
Mutation carriers risk management options include salpingo-oophorectomy, risk reducing mastectomy, annual cancer screening and chemoprevention.
For BRCA1 and for BRCA2 patients who undergo salpingo-oophorectomy decreases the risk of both breast cancer and ovarian cancers.
Oophorectomy for a patient who is BRCA1 between the ages of 35 and 40 years and before age 45 years with women with BRCA 2 sequence variations, is associated with a significant reduction in all cause mortality (Breast Cancer Clinical Study Group).
A history of breastfeeding is associated with a 23 percent reduction in ovarian cancer risk among women who carry BRCA1 or BRCA2 mutations.
Proportion of families in the U.S. with high risk disease associated with BRCA1 mutations range from 16-39% and BRCA2 mutations account for 19-25% of families.
More than 300,000 women in the US estimated to carry or BRCA2 mutation genes.
With these gene mutations women inherit a 5-20 fold increased risk of developing breast and ovarian cancer.
Genes associated with 5-10% of breast cancers.
MRI twice as sensitive as mammography in detecting malignancies in women with a genetic susceptibility to breast cancer.
Risk reducing salpingo-oophorectomy reduces the risk of ovarian, fallopian tube and primary peritoneal cancers, and if performed premenopausally, also reduces the risk risk of BRCA associated breast cancer (Kauff ND, Rebbeck TR)
BRCA1 mutation carriers may have differential benefits from preventive interventions compared with BRCA2 mutation carriers (Kauff KD et al).
Selective ogen reception modulators may reduce the risk of estrogen receptor positive inherited breast cancer.
Short-term hormonal therapy with estrogen alone or estrogen and progesterone in patients who have a BRCA mutation is not associated with increase risk of breast cancer, and factor may have an associated decreased risk.
Oral contraceptives decreases the risk of BRCA associated gynecologic cancer, but it may increase breast cancer risk (Narod SA, Brohet RM).
In a metaanalysis involving 1100 women with a BRCA mutation and intact breasts who underwent risk reduction bilateral salpingooophorectomy before the onset of natural menopause, no excess risk was found to be associated with the use of hormone therapy beyond baseline increasing the risk of breast cancer for carriers of mutated BRAC1BRCA2.
MRI breast screening can detect twice as many cancers as does simultaneously performed mammography and results in interval cancer rates below 10% in patients with BRCA mutations.
In BRCA mutation carriers MRI can detect early breast cancers with 75-94% of screen detected invasive tumors being 2 cm or smaller at diagnosis and 75-83% being negative for axillary node metastases (Kriege M, Kuhl, CK).
A substantial gain in life expectancy of 2.9-5.3 years results from risk reducing mastectomy and gains of 0.3-1.7 years can be achieved with risk reducing salpingo-oophorectomy, depending upon the level of cancer risk and in young women with BRCA1 mutations (Schrag D) in an.
Prophylactic mastectomies confer a 90% risk reduction in breast cancer.
Prophylactic oophorectomy reduces ovarian cancer risk by 50%.
Prophylactic bilateral salpingo-oophorectomy is recommended for ovarian cancer risk reduction to be done by age 40.
In a retrospective study 493 BRCA1/2 mutation carriers undergoing prophylactic bilateral salpingo-oophorectomy there was a 98% reduction in ovarian, fallopian tube, and primary peritoneal cancers (Boyd J).
Risk of ovarian cancer decreases with each birth but not with increased duration of use of oral contraceptives.
Patients with invasive ovarian carcinoma with BRCA1 or BRCA2 genes have improved 5 year survival, with BRCA2 patients having the best prognosis (Bolton KL et al).
Lifetime risk of ovarian cancer estimated to be 36-60% in BRCA1 mutations and 16-27% in BRCA2 mutations.
BRCA1 mutation carriers tend to develop ovarian cancer approximately 8 years earlier than BRCA2 mutation carriers.
Mutations occur in high risk families of African ancestry with 28% testing positive for a deleterious mutation in 1 of these genes.
Mutations increase susceptibility to cancers of the ovary, fallopian tube and peritoneum.
Mammography sensitivity is poor, ranging from 36-55% (Gui).
Mammography in patients with BRAC 1 and BRAC 2 mutations have a relatively rapid doubling time, and interval cancers diagnosed between yearly mammography screenings have been noted in approximately 50% of these women.
Patients with BRCA mutation carriers can present with relatively benign radiographic features contributing to false negative interpretations.
Prophylactic mastectomy at age 25+ prophylactic oophorectomy at age 40 years maximize his survival probability in patients with BRCA1/BRCA2 gene mutations: Substituting mammography plus MRI screening for prophylactic mastectomy offers comparable survival (Kurian A).
In the Monte Carlo model is estimated that survival probability of a patient with a BRCA1 mutation by age 70 is 53% for patients with BRCA 2 mutation carriers it is 71%(Kurian A).
The most effective single intervention for BRCA1 mutation carriers is prophylactic oophorectomy at age 40 yielding a 15% absolute survival gain and 4 BRCA2 mutation carriers the most effective single intervention is prophylactic mastectomy with a 7% survival gain if performed at age 40 (Kurian A).
Patients with high-penetrance heritable mutations in either BRCA1 or BRCA2 gene have a 30% risk of cancer in the contralateral breast and in increased risk of ipsilateral recurrence.
The risk of subsequent contralateral breast cancer in BRCA carriers is markedly increased, reported to be as high as 25%–30% over 10 years and more than 40% over 15 years, as compared with 3% and 7%, respectively, in non-carriers.
The risk of a second cancer in the contralateral breast within 10 years maybe his is 40-50% in some series and contralateral prophylactic mastectomy for maximal risk reduction against a second breast cancer needs to be considered (Metcalfe K).
PARP proteins our family of nuclear enzymes involved in multiple DNA damage pathways.
PARP1 is the most involved in DNA repair. parp.vines to DNA brakes, recruits DNA damage repair components that cooperate to stabilize the replication, fork, and allows for DNA repair.
Olaparib is efficacious for patients with BRCA mutation breast cancer-improves median progression free survival in patients with such mutations compared to chemotherapy.
PARP inhibitors in advanced or metastatic BRCA positive breast cancer treatment improves medium progression free survival of approximately three months, but does not improve overall survival compared to chemotherapy.
