 Birt–Hogg–Dubé syndrome (BHD) is an autosomal dominant genetic disorder that can cause susceptibility to kidney cancer, renal and pulmonary cysts, and noncancerous tumors of the hair follicles, called fibrofolliculomas.
Birt–Hogg–Dubé syndrome (BHD) is an autosomal dominant genetic disorder that can cause susceptibility to kidney cancer, renal and pulmonary cysts, and noncancerous tumors of the hair follicles, called fibrofolliculomas.
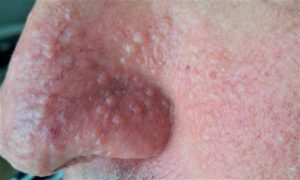
Fibrofolliculomas are the most common manifestation, found on the face and upper trunk in over 80% of patients with BHD over the age of 40.
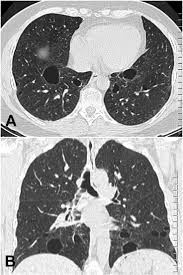
Pulmonary cysts occur in 84% of patients.
Spontaneous pneumothorax occurs in 24% of people with BHD.
Kidney tumors, both cancerous and benign, occur in 14–34% of people with BHD: kidney cancers are often rare hybrid tumors.
Any of these conditions in a family suggest a a diagnosis of Birt–Hogg–Dubé syndrome.
Diagnosis is confirmed by a genetic test for a mutation in the FLCN gene, which codes for the protein folliculin.
The FLCN gene appears to be a tumor suppressor gene that restricts cell growth and division.
FLCN mutations are detected by sequencing in 88% of probands with this syndrome.
The folliculin (FLCN) gene is a 14-exon gene is located on the short arm of chromosome 17 (17p11.2) and has a cytosine-rich region in exon 11 particularly susceptible to mutation.
FLCN creates a protein, folliculin, that has two isoforms.
It appears to act as a tumor suppressor, and is expressed strongly in the skin, distal nephrons, and type I pneumocytes.
The most common mutation in this region is the insertion or deletion of a cytosine residue, found in 53% of BHD-affected families.
The mutations in the FLCN gene that cause Birt–Hogg–Dubé syndrome are germline mutations, which means that they occur in every cell of the body and can be passed down to future generations in an autosomal dominant fashion, but can occur as a new mutation in an individual with no prior family history
These mutations are often passed from one generation to the next in an autosomal dominant fashion, but can occur as a new mutation in an individual with no prior family history, a de novo mutation.
BHD has very high penetrance.
BHD has similarities to other diseases, which must be ruled out when making a diagnosis: tuberous sclerosis, which causes skin lesions similar to fibrofolliculomas, and Von Hippel–Lindau disease, which causes hereditary kidney cancers.
Patients with BHD are treated preventatively, with monitoring of kidneys and lungs using medical imaging.
Birt–Hogg–Dubé syndrome affects the skin and increases the risk of tumors in the kidneys and lungs.
It is is characterized by multiple noncancerous, dome-shaped tumors of the hair follicles, fibrofolliculomas, particularly on the face, neck, and more rarely, the upper chest.
Fibrofolliculomas have an opaque white color or a yellowish tone with a waxy, smooth texture.
Fibrofolliculoma tumors are always found on and around the nose and on and behind the outer ear.
Typically, fibrofolliculomas appear in a person’s 20s or 30s, and are found in more than 80% of people with the syndrome above the age of 40.
The tumors become larger and more numerous over time, and differ between individuals.
Fibrofolliculomas may appear as plaques, look similar to a comedo with a plug of keratin, or include epidermoid cysts.
Other tumors can include tumors of the hair disc, which may be identical to fibrofolliculomas, angiofibromas, and perifollicular fibromas.
About 40% of people or families with the disease have papules in their mouths: cheek buccal mucosa, tongue, gums, or lips.
Many patients with BHD have skin lesions that appear to be skin tags, but may instead be fibrofolliculomas.
These lesions are usually found in the armpit, on the eyelids, and in folds of skin.
Not all individuals develop the facial tumors; some families with the mutation that causes BHD develop only kidney tumors or spontaneous pneumothorax.
A chromophobe renal cell carcinoma, the second-most common cancer associated with BHD.
People over 20 years of age with BHD have an increased risk of developing slow-growing kidney tumors: chromophobe renal carcinoma and renal oncocytoma, respectively, kidney cysts, and possibly tumors in other organs and tissues.
These renal tumors often occur in both kidneys and in multiple locations in each kidney.
The average number of kidney tumors found in a person with BHD is 5.3
Up to 28 kidney tumors have been found in patients with BHD.
Hybrid oncocytoma/chromophobe carcinomas, are found in 50% of cases, are the most commonly found cancer, followed by chromophobe renal carcinoma, clear cell renal carcinoma, renal oncocytoma, and papillary renal cell carcinoma.
Patients with BHD over 40 years old and men are more likely to develop kidney tumors, which are diagnosed at a median age of 48.
Kidney cancer associated with BHD have been diagnosed in people at ages as young as 20.
Patients with BHD are at roughly at seven times the risk of kidney cancer compared to the unaffected population: the incidence among people with the disease range from 14 to 34%.
Rarely, it is associated with clear cell renal cell carcinoma and papillary renal cell carcinoma.
BHD related renal cell carcinoma occurs later in life and has a poor prognosis.
Most types of tumors typically associated with BHD are considered less aggressive.
However, cases of advanced or metastatic kidney cancer have been observed in people with the syndrome.
Both benign and cancerous tumors in BHD can reduce kidney function over time as they grow larger.
BHD associated with fibrofolliculomas, kidney tumors, and frequently develop cysts in the subpleural lung base or intraparenchymal space that may rupture and cause a pneumothorax.
The cysts do not cause other pulmonary symptoms and lung function is usually normal.
More than 83% of people with BHD have pulmonary cysts, without chronic obstructive pulmonary disease or generalized respiratory failure.
BHD it does cause emphysema.
Around 24% of people with the disease have at least one spontaneous pneumothorax, 30 times the occurrence in unaffected people.
Pneumothorax caused by BHD often occurs in middle age, but 17% of affected people have a spontaneous pneumothorax before turning 40.
Pneumothorax have been seen in people as young as 7.
BHD occurs in some families affecting the lungs only.
Thyroid nodules are present in 65% of individuals and 90% of families with the BHD syndrome.
Other associated findings:
multinodular goiter, medullary thyroid carcinoma, parotid oncocytoma, colonic polyposis, connective tissue nevus, lipomas, angiolipomas, parathyroid adenomas, flecked chorioretinopathy, neurothekeoma, meningiomas, angiofibromas of the face, trichoblastomas, cutaneous focal mucinosis, cutaneous leiomyoma, breast cancer, tonsillar cancer, colorectal cancer, sarcoma of the leg, lung cancer, melanoma, dermatofibrosarcoma protuberans, basal cell carcinoma, cutaneous leiomyosarcoma, and squamous cell carcinoma.
Folliculin is found in the parotid gland, brain, breast, pancreas, prostate, and ovaries.
Mutations in the FLCN gene may interfere with the ability of folliculin to restrain cell growth and division.
Mutations in the FLCN gene may lead to the formation of noncancerous and cancerous tumors.
Folliculin functions through its involvement with cellular metabolism, possibly by modulating the mTOR (mammalian target of rapamycin) pathway and/or oxidative phosphorylation in mitochondria.
Folliculin forms a complex with AMP-activated protein kinase.
Folliculin’s participation in the mTOR pathway clarifies the similarity in phenotype between BHD syndrome, Cowden syndrome, tuberous sclerosis, and Peutz–Jeghers syndrome.
The C-terminal end of folliculin interacts with FNIP1, and thereby possibly the mTOR pathway.
The 508th amino acid, normally lysine, in follicular is affected by a missense mutation in some people with BHD.
People with BHD have one mutated copy of the FLCN gene in each cell, and haploinsufficiency is enough to cause the fibrofolliculomas and pulmonary cysts.
Haploinsufficiency with one copy of the gene keeps kidney cells in check.
However, random mutations may inactivate the normal copy of the gene in a subset of cells, resulting in cells that have no functional copies of the FLCN gene: allowing the cells grow out of control
This loss of heterozygosity is a common mechanism in cancer, and it is frequently detected in the renal cancers associated with BHD.
BHD-associated renal tumorigenesis differs between the kidney, where loss of FLCN heterozygosity is responsible for cancers, and the skin, where FLCN is strongly expressed in heterozygotes.
FLCN has isb overexpressed in fibrofolliculoma tissue, and to have very low levels of expression in affected kidneys.
The mTOR pathway is shown to be activated in renal tumor tissue.
Diagnosis
BHD is suggested by clinical findings but is definitively diagnosed by molecular genetic testing to detect mutations in the FLCN gene.
The classical clinical triad includes benign growths of the hair follicles, pulmonary cysts and spontaneous pneumothorax, and bilateral, multifocal renal tumors.
The cutaneous manifestations: fibrofolliculomas, trichodiscomas which are hamartomatous lesions with a hair follicle often found on the face, and skin tags.
Most individuals with BHD are found to have multiple cysts in both lungs, and 24% have had one or more episodes of pneumothorax.
Renal tumors can manifest as multiple types of renal cell carcinoma, but certain pathological subtypes: chromophobe, oncocytoma, and oncocytic hybrid tumors are more commonly seen.
Patients with BHD may only manifest the pulmonary and/or renal findings, without any skin lesions.
Genetic testing can confirm the clinical diagnosis and determines other at-risk individuals in a family even if they have not yet developed BHD symptoms.
Differentiatial diagnosis:
Tuberous sclerosis, von Hippel–Lindau disease, hereditary papillary renal cancer and hereditary leiomyomatosis and renal cell cancer syndrome, Marfan syndrome, Ehlers–Danlos syndrome, tuberous sclerosis complex, alpha1-antitrypsin deficiency, and cystic fibrosis, Langerhans cell histiocytosis and lymphangioleiomyomatosis
Diseases that can mimic the dermatologic manifestations of BHD, including tuberous sclerosis complex, Cowden syndrome, familial trichoepitheliomas, and multiple endocrine neoplasia type 1.
Management: The fibrofolliculomas can be removed surgically, through curettage, shave excision, skin resurfacing, or laser ablation.
These tumors often recur.
Thyroid/parotid ultrasound should be considered annually.
CT scans, ultrasounds, or MRIs of the kidneys are recommended regularly, and family members are advised not to smoke.
MRIs are the preferred method for surveillance of the kidneys in people with BHD because of no radiation and are more sensitive than ultrasounds.
Nephrectomy is sometimes indicated, but in cases of BHD, partial nephrectomy may be successful.
The disorder has been reported in more than 100 to 400 families, and it is inherited in an autosomal dominant pattern.
It is under-diagnosed because of the variability in its expression.
The pattern of mutations and spectrum of symptoms are heterogeneous.
Less severe skin phenotypes are seen in women and people of both sexes who have a late onset of skin symptoms.
