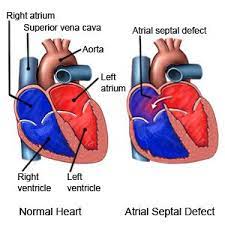
 Atrial septal defect (ASD) is a congenital heart defect in which blood flows between the atria of the heart.
Atrial septal defect (ASD) is a congenital heart defect in which blood flows between the atria of the heart.
Some flow is a normal condition both pre-birth and immediately post-birth via the foramen ovale.
When it does not naturally close after birth it is referred to as a patent foramen ovale (PFO).
After PFO closure the atria normally are separated by a dividing wall, the interatrial septum.
As a group, atrial septal defects are detected in one child per 1500 live births.
PFOs are quite common appearing in 10–20% of adults, but when asymptomatic go undiagnosed.
ASDs make up 30 to 40% of all congenital heart diseases that are seen in adults.
The ostium secundum atrial septal defect accounts for 7% of all congenital heart lesions.
This lesion shows a male:female ratio of 1:2.
If this septum is defective or absent, then oxygen-rich blood can flow from one side of the heart depending on whether the left or right atrium has the higher blood pressure.
The left atrium has the higher pressure, in the absence of other heart defects.
An ASD can lead to lower-than-normal oxygen levels in the arterial blood that supplies the brain, organs, and tissues.
An ASD may not produce noticeable signs or symptoms if the defect is small.
People who have had a cryptogenic stroke are more likely to have a PFO than the general population.
The amount of shunting present determines the hemodynamic significance of the ASD.
A right-to-left-shunt results in venous blood entering the left side of the heart and into the arterial circulation without passing through the pulmonary circulation to be oxygenated: cyanosis may result.
A hole in the septum called the foramen ovale allows blood from the right atrium to enter the left atrium during fetal development.
The foramen ovale allows blood to bypass the nonfunctional fetal lungs while the fetus obtains its oxygen from the placenta.
During fetal development the septum primum acts as a valve over the foramen ovale
With birth, the pressure in the right side of the heart drops as the lungs open causing the foramen ovale to close entirely.
However, in about 25% of adults, the foramen ovale does not entirely seal closed.
Any elevation of the pressure in the pulmonary circulatory system can cause the foramen ovale to remain open.
There are six types of atrial septal defects that are differentiated from each other by whether they involve other structures of the heart and how they are formed during the developmental process during early fetal development.
The ostium secundum atrial septal defect is the most common type of atrial septal defect and comprises 6–10% of all congenital heart diseases.
It involves a patent ostium secundum.
The secundum atrial septal defect usually arises from an enlarged foramen ovale, inadequate growth of the septum secundum, or excessive absorption of the septum primum.
About 10 to 20% of individuals with ostium secundum ASDs also have mitral valve prolapse.
An ostium secundum ASD accompanied by an acquired mitral valve stenosis is called Lutembacher’s syndrome.
Most individuals with an uncorrected secundum ASD do not have significant symptoms through early adulthood.
More than 70% develop symptoms by about 40 years of age.
Symptoms are typically decreased exercise tolerance, easy fatigability, palpitations, and syncope.
Complications of an uncorrected secundum ASD include pulmonary hypertension, right-sided congestive heart failure.
While pulmonary hypertension is unusual before 20 years of age.
Pulmonary hypertension is seen in 50% of individuals above the age of 40.
Progression of LS to Eisenmenger’s syndrome occurs in 5 to 10% of individuals late in the disease process.
PFO is linked to stroke, sleep apnea, migraine with aura, cluster headache, decompression sickness, Raynaud’s phenomenon, hyperventilation syndrome, transient global amnesia (TGA), and leftsided carcinoid heartdisease (mitral valve).
No cause is established for a foramen ovale to remain open:
heredity and genetics may play a role.
The mechanism by which a PFO may play a role in stroke is called paradoxical embolism: a blood clot from the venous circulatory system is able to pass from the right atrium directly into the left atrium via the PFO, rather than being filtered by the lungs, and thereupon into systemic circulation toward the brain.
PFO is common in patients with an atrial septal aneurysm (ASA), a much rarer condition, which is also linked to cryptogenic stroke.
While PFO is present in 25% in the general population, the probability of someone having a PFO increases to about 40 to 50% in those who have had a cryptogenic stroke.
A defect in the ostium primum is occasionally classified as an atrial septal defect, but it is more commonly classified as an atrioventricular septal defect.
Ostium primum defects are less common than ostium secundum defects.
This type of defect is usually associated with Down syndrome.
A sinus venosus ASD has a defect involving the venous inflow of either the superior vena cava or the inferior vena cava.
A sinus venosus ASD that involves the superior vena cava makes up 2 to 3% of all interatrial communication.
A sinus venosus atrial septal defect is a type of atrial septal defect primarily associated with the sinus venosus.
They represent 5% of atrial septal defects, and can occur near the superior vena cava or inferior vena cava, but the former are more common.
A sinus venosus ASD is located at the junction of the superior vena cava and the right atrium, and is frequently associated with anomalous drainage of the right-sided pulmonary veins into the right atrium, instead of the normal drainage of the pulmonary veins into the left atrium.
The interatrial septum can be divided into five septal zones. If the defect involves two or more of the septal zones, then the defect is termed a mixed atrial septal defect.
Disease entities or complications from ASD: cardiac arrhythmia, as well as more frequent respiratory infections.
Eisenmenger’s syndrome:
If a net flow of blood exists from the left atrium to the right atrium, called a left-to-right shunt, then an increase in the blood flow through the lungs.
Eisenmenger’s syndrome, a rare and late complication of an ASD.
Paradoxical embolus
Migraine?
Down syndrome – patients with Down syndrome have higher rates of ASDs, especially a particular type that involves the ventricular wall.
As many as one half of Down syndrome patients have some type of septal defect.
Ebstein’s anomaly– about 50% of individuals with Ebstein anomaly have an associated shunt between the right and left atria, either an atrial septal defect or a patent foramen ovale.
Fetal alcohol syndrome – about one in four patients with fetal alcohol syndrome has either an ASD or a ventricular septal defect.
Holt–Oram syndrome – both the osteium secundum and osteum primum types of ASD are associated with Holt–Oram syndrome.
Lutembacher’s syndrome – the presence of a congenital ASD along with acquired mitral stenosis.
In unaffected individuals, the left ventricle has to produce enough pressure to pump blood throughout the entire body, while the right ventricle needs only to produce enough pressure to pump blood to the lungs.
A large ASD (> 9 mm), may result in a clinically remarkable left-to-right shunt: blood shunts from the left atrium to the right atrium.
This blood from the left atrium may cause a volume overload of both the right atrium and the right ventricle.
This condition can result in enlargement of the right side of the heart and ultimately heart failure.
Processes that increase the pressure in the left ventricle can cause worsening of the left-to-right shunt: hypertension, which increases the pressure that the left ventricle has to generate to open the aortic valve during ventricular systole, and coronary artery disease which increases the stiffness of the left ventricle, thereby increasing the filling pressure of the left ventricle during ventricular diastole.
As the left-to-right shunt increases the filling pressure of the right heart it forces the right ventricle to pump out more blood than the left ventricle.
This constant overloading of the right side of the heart causes an overload of the entire pulmonary vasculature, and pulmonary hypertension may develop.
The right ventricle is forced to generate higher pressures to try to overcome the pulmonary hypertension, and may lead to right ventricular failure.
If the ASD is left uncorrected, the pulmonary hypertension progresses and the pressure in the right side of the heart becomes greater than the left side of the heart : resulting in a right-to-left shunt.
A right-to-left shunt, that results, is known as Eisenmenger’s syndrome.
With the right-to-left shunting a portion of the oxygen-poor blood gets shunted to the left side of the heart and ejected to the peripheral vascular system, causing cyanosis.
DIAGNOSIS:
Most patients with a significant ASD are diagnosed in utero or in early childhood with the use of ultrasonography or auscultation of the heart sounds during physical examination.
Some individuals with an ASD require surgical correction of their ASD during childhood.
The signs and symptoms due to an ASD are related to the size of the intracardiac shunt: larger shunts associated with symptoms at a younger age.
Adults with an uncorrected ASD symptoms include dyspnea on exertion, congestive heart failure, stroke.
Uncorrected ASD may be associated
with an abnormal chest X-ray or an abnormal ECG and may have atrial fibrillation.
If the ASD causes a left-to-right shunt, the pulmonary vasculature in both lungs may appear dilated on chest X-ray, due to the increase in pulmonary blood flow.
Physical examination findings in an adult with an ASD include findings due to intracardiac shunt and those that are secondary to the right heart failure that may be present.
Respiratory variations occur in the splitting of the second heart sound (S2).
During respiratory inspiration, the negative intrathoracic pressure causes increased blood return into the right side of the heart, causing the pulmonic valve to stay open longer during ventricular systole.
Respiratory inspiration and negative intrathoracic pressure causes a normal delay in the P2 component of second sound.
During expiration, the positive intrathoracic pressure causes decreased blood return to the right side of the heart, allowing the pulmonic valve to close earlier at the end of ventricular systole, causing P2 to occur earlier.
With ASD, there is a fixed splitting of S2 because the extra blood return during inspiration gets equalized between the left and right atria due to the communication that exists between the atria.
The right ventricle can be thought of as continuously overloaded because of the left-to-right shunt, producing a widely split S2.
In transthoracic echocardiography, an atrial septal defect may be seen as a jet of blood from the left atrium to the right atrium.
Better visualization of the atria is achieved with transesophageal echocardiography, and this test may be performed in individuals with a suspected ASD which is not visualized on transthoracic imaging.
A method for detecting a PFO or other ASDs other than transesophagal ultrasound is transcranial Doppler with bubble contrast: revealing the cerebral impact of the ASD or PFO.
The ECG findings in atrial septal defect vary with the type of defect present.
There may be a prolonged PR interval (a first-degree heart block).
The prolongation of the PR interval is probably due to the enlargement of the atria common in ASDs and the increased distance due to the defect, increasing the distance of internodal conduction from the SA node to the AV node.
Patients with a primum ASD have a left axis deviation of the QRS complex, while those with a secundum ASD have a right axis deviation of the QRS complex.
Individuals with a sinus venosus ASD exhibit a left axis deviation of the P wave.
A common finding in the ECG is the presence of incomplete right bundle branch block, which is so characteristic that if it is absent, the diagnosis of ASD should be reconsidered.
TREATMENT:
Most patients with a PFO are asymptomatic and do not require any specific treatment.
Cryptogenic mechanism for stroke is such individuals is likely embolic due to paradoxical emboli, a left atrial appendage clot, a clot on the inter-atrial septum, or within the PFO tunnel.
The Amplatzer Septal Occluder is a device specifically designed to close an ASD.
Percutaneous PFO closure in addition to antiplatelet therapy is suggested for all who meet all the following criteria:
Age ≤ 60 years at onset of first stroke,
Embolic-appearing cryptogenic ischemic stroke
PFO with a right-to-left interatrial shunt detected by bubble study
A variety of PFO closure devices may be implanted via catheter-based procedures.
PFO closure is more effective at reducing recurrent ischemic stroke when compared to medical therapy.
There is limited data on the effectiveness of anticoagulation in reducing stroke in this population, it is hypothesized anticoagulation should be superior to antiplatelet therapy at reducing risk of recurrent stroke.
If the atrial septal defect is causing the right ventricle to enlarge a secundum atrial septal defect should generally be closed.
Otherwise, observation every two or three years is recommended.
Closure of an ASD include surgical closure and percutaneous closure.
Prior to correction of an ASD, an evaluation is made of the severity of pulmonary hypertension, if present. Com
If pulmonary hypertension is present, the evaluation may include a right heart catheterization, and measuring pressures and oxygen saturations in the superior vena cava, inferior vena cava, right atrium, right ventricle, and pulmonary artery, and in the wedge position.
Individuals with a pulmonary vascular resistance (PVR) less than 7 wood units show regression of symptoms after ASDA closure.
However, individuals with a PVR greater than 15 wood units have increased mortality associated with closure of the ASD.
If the pulmonary arterial pressure is more than two-thirds of the systemic systolic pressure, a net left-to-right shunt should occur.
If Eisenmenger’s physiology has set in, the right-to-left shunt must be shown to be reversible with pulmonary artery vasodilators prior to surgery.
Surgical mortality due to closure of an ASD is lowest when the procedure is performed prior to the development of significant pulmonary hypertension.
The lowest mortality rates for ASD closure are achieved in individuals with a pulmonary artery systolic pressure less than 40 mmHg.
If Eisenmenger’s syndrome has occurred, a significant risk of mortality exists, as the pressure in the right ventricle has raised high enough to reverse the shunt in the atria.
If the ASD is then closed, the afterload that the right ventricle has to act against has suddenly increased, and may cause immediate right ventricular failure, since it may not be able to pump the blood against the pulmonary hypertension.
Surgical closure of an ASD involves opening up at least one atrium and closing the defect with a patch.
Percutaneous device closure involves the passage of a catheter into the heart through the femoral vein guided by fluoroscopy and echocardiography.
The percutaneous has discs that can expand to a variety of diameters at the end of the catheter.
The percutaneous catheter is placed in the right femoral vein and guided into the right atrium.
The device is inserted into the septal wall.
This type of PFO closure is more effective than drug or other medical therapies for decreasing the risk of future thromboembolism.
The most common adverse effect of this device closure is new-onset atrial fibrillation.
Percutaneous device closure other complications, all rare, include device migration, erosion and embolization and device thrombosis or formation of an inflammatory mass with risk for recurrent ischemic stroke.
Percutaneous closure of an ASD is currently only indicated for the closure of secundum ASDs.
There must be a sufficient rim of tissue around the septal defect so that the closure device does not impinge upon the superior vena cava, inferior vena cava, or the tricuspid or mitral valves.
The Amplatzer Septal Occluder (ASO) is commonly used to close ASDs.
Percutaneous closure is the method of choice in most centers.
As a group, atrial septal defects are detected in one child per 1500 live births.
PFOs are quite common appearing in 10–20% of adults, but when asymptomatic go undiagnosed.
ASDs make up 30 to 40% of all congenital heart diseases that are seen in adults.
The ostium secundum atrial septal defect accounts for 7% of all congenital heart lesions.
This lesion shows a male:female ratio of 1:2.
Complications of an uncorrected secundum ASD include pulmonary hypertension, right-sided congestive heart failure.
A sinus venosus ASD that involves the superior vena cava makes up 2 to 3% of all interatrial communication, and is located at the junction of the superior vena cava and the right atrium.
It is frequently associated with anomalous drainage of the right-sided pulmonary veins into the right atrium, instead of the normal drainage of the pulmonary veins into the left atrium.
Patients with an uncorrected atrial septal defect may be at increased risk for developing a cardiac arrhythmia, and more frequent respiratory infections.
Eisenmenger’s syndrome
If a net flow of blood exists from the left atrium to the right atrium, a left-to-right shunt, then an increase in the blood flow through the lungs happens.
If the increased blood flow persists, the pulmonary blood vessels may stiffen, causing pulmonary hypertension, which increases the pressures in the right side of the heart, leading to the reversal of the shunt into a right-to-left shunt.
If a reversal of the shunt occurs, the blood flowing in the opposite direction through the ASD is called Eisenmenger’s syndrome, a rare and late complication of an ASD.
Venous thrombus embolizations normally go to the lung and cause pulmonary emboli: In an individual with ASD, these emboli can potentially enter the arterial system, which can cause any phenomenon attributed to acute loss of blood to a portion of the body, including cerebrovascular accident, infarction of the spleen or intestines, or even a distal extremity.
This is as a paradoxical embolus because the clot material paradoxically enters the arterial system instead of going to the lungs.
Research suggests that a proportion of cases of migraine may be caused by PFO, and closure of a PFO can reduce symptoms in certain cases.
This remains controversial; 20% of the general population has a PFO, which for the most part, is asymptomatic.
About 20% of the female population has migraines, and the placebo effect in migraine typically averages around 40%. The high frequency of these facts make finding statistically significant relationships between PFO and migraine difficult.
In a large randomized controlled trial, migraine headache cessation was not more prevalent in the group of migraine patients who underwent closure of their PFOs.
Patients with Down syndrome have higher rates of ASDs, especially a particular type that involves the ventricular wall.
As many as one half of Down syndrome patients have some type of septal defect.
Ebstein’s anomaly– about 50% of individuals with Ebstein anomaly have an associated shunt between the right and left atria, either an atrial septal defect or a patent foramen ovale.
Fetal alcohol syndrome – about one in four patients with fetal alcohol syndrome has either an ASD or a ventricular septal defect.
In the case of a large ASD (> 9 mm), which may result in a clinically remarkable left-to-right shunt, blood shunts from the left atrium to the right atrium.
This extra blood from the left atrium may cause a volume overload of both the right atrium and the right ventricle, and if not treated this condition can result in enlargement of the right side of the heart and ultimately heart failure.
Any process that increases the pressure in the left ventricle can cause worsening of the left-to-right shunt: hypertension increases the pressure that the left ventricle has to generate to open the aortic valve during ventricular systole, and coronary artery disease which increases the stiffness of the left ventricle, thereby increasing the filling pressure of the left ventricle during ventricular diastole.
The left-to-right shunt increases the filling pressure of the right heart (preload) and forces the right ventricle to pump out more blood than the left ventricle.
This constant overloading of the right side of the heart causes an overload of the entire pulmonary vasculature, eventually, causing pulmonary hypertension to develop.
As the right ventricle is forced to generate higher pressures to try to overcome the pulmonary hypertension it leads to right ventricular failure.
If the ASD is left uncorrected, the pulmonary hypertension progresses and the pressure in the right side of the heart becomes greater than the left side of the heart, reversing the pressure gradient across the ASD causes the shunt to reverse – a right-to-left shunt.
This phenomenon is known as Eisenmenger’s syndrome.
Once right-to-left shunting occurs, a portion of the oxygen-poor blood gets shunted to the left side of the heart and ejected to the peripheral vascular system, causing signs of cyanosis.
Diagnosis:
Most individuals with a significant ASD are diagnosed in utero or in early childhood with the use of ultrasonography or auscultation of the heart sounds during physical examination.
Some individuals with an ASD have surgical correction of their ASD during childhood.
The signs and symptoms due to an ASD are related to the size of the intracardiac shunt.
Individuals with a larger shunt tend to present with symptoms at a younger age.
With an uncorrected ASD adult patients present with symptoms of dyspnea on exertion congestive heart failure, or cerebrovascular accident (stroke).
They may have an nabnormal chest X-ray or an abnormal ECG and may have atrial fibrillation.
If the ASD causes a left-to-right shunt, the pulmonary vasculature in both lungs may appear dilated on chest X-ray, due to the increase in pulmonary blood flow.
Physical exam findings in an adult with an ASD include: those related directly to the intracardiac shunt and those that are secondary to the right heart failure that may be present in these individuals.
In unaffected individuals, respiratory variations occur with the splitting of the second heart sound.
During respiratory inspiration, the negative intrathoracic pressure causes increased blood return into the right side of the heart, causing the pulmonic valve to stay open longer during ventricular systole: This results in a normal delay in the P2 component of S2.
During expiration, the positive intrathoracic pressure causes decreased blood return to the right side of the heart, and the reduced volume in the right ventricle allows the pulmonic valve to close earlier at the end of ventricular systole, causing P2 to occur earlier.
In individuals with an ASD, there is a fixed splitting of S2 because the extra blood return during inspiration gets equalized between the left and right atria due to the communication that exists between the atria in individuals with ASD.
The right ventricle is continuously overloaded because of the left-to-right shunt, producing a widely split S2.
Because the atria are linked via the atrial septal defect, inspiration produces no net pressure change between them, and has no effect on the splitting of S2.
Thus, S2 is split is said to be fixed.
In transthoracic echocardiography, an atrial septal defect may be seen on imaging as a jet of blood from the left atrium to the right atrium.
The shunt fraction can be estimated using echocardiography.
The ECG findings in atrial septal defect vary: prolonged PR interval, a first-degree heart block, probably due to the enlargement of the atria common in ASDs and the increased distance due to the defect itself.
Both of these can cause an increased distance of internodal conduction from the SA node to the AV node.
In addition to the PR prolongation, individuals with a primum ASD have a left axis deviation of the QRS complex, while those with a secundum ASD have a right axis deviation of the QRS complex.
Individuals with a sinus venosus ASD exhibit a left axis deviation of the P wave, and not the QRS complex.
A common finding in the ECG is the presence of incomplete right bundle branch block:if it is absent, the diagnosis of ASD should be reconsidered.
Most patients with a PFO are asymptomatic and do not require any specific treatment.
Those who develop a stroke require further workup to identify the etiology.
In those where an obvious etiology is not identified, they are defined as having a cryptogenic stroke, likely a an embolic due to paradoxical emboli, a left atrial appendage clot, a clot on the inter-atrial septum, or within the PFO tunnel.
The Amplatzer Septal Occluder is a device specifically designed to close an ASD.
Until recently, patients with PFO and cryptogenic stroke were treated with antiplatelet therapy only.
Previous studies did not identify a clear benefit of PFO closure over antiplatelet therapy in reducing recurrent ischemic stroke.
Presently, percutaneous PFO closure in addition to antiplatelet therapy is suggested for all who meet all the following criteria:
Age ≤ 60 years at onset of first stroke,
Embolic-appearing cryptogenic ischemic stroke PFO with a right-to-left interatrial shunt detected by bubble study
A variety of PFO closure devices may be implanted via catheter-based procedures.
PFO closure is more effective at reducing recurrent ischemic stroke when compared to medical therapy. In most of these studies, antiplatelet and anticoagulation were combined in the medical therapy arm.
Literature supports the recommendation of anticoagulation over the use of antiplatelet therapy in patients with PFO and cryptogenic stroke.
However, more evidence is required comparing of PFO closure with anticoagulation or anticoagulation with antiplatelet therapy.
If the atrial septal defect is causing the right ventricle to enlarge a secundum atrial septal defect should be closed.
If the ASD is not causing problems the defect may simply be checked every two or three years.
Methods of closure of an ASD include surgical closure and percutaneous closure.
Prior to correction of an ASD, an evaluation is made of the severity of the individual’s pulmonary hypertension, if present, and whether it is reversible.
Pulmonary hypertension is not always present in adults who are diagnosed with an ASD in adulthood.
If pulmonary hypertension is present, the evaluation may include a right heart catheterization, and measuring pressures and oxygen saturations in the superior vena cava, inferior vena cava, right atrium, right ventricle, and pulmonary artery, and in the wedge position.
Individuals with a pulmonary vascular resistance (PVR) less than 7 wood units show regression of symptoms, however , individuals with a PVR greater than 15 wood units have increased mortality associated with closure of the ASD.
If the pulmonary arterial pressure is more than two-thirds of the systemic systolic pressure, a net left-to-right shunt should occur at least 1.5:1 or evidence of reversibility of the shunt when given pulmonary artery vasodilators prior to surgery.
Surgical mortality due to closure of an ASD is lowest when the procedure is performed prior to the development of significant pulmonary hypertension: the lowest mortality rates are achieved in individuals with a pulmonary artery systolic pressure less than 40 mmHg.
If Eisenmenger’s syndrome has occurred, a significant risk of mortality exists regardless of the method of closure of the ASD.
In patients that have developed Eisenmenger’s syndrome, the pressure in the right ventricle has raised high enough to reverse the shunt in the atria.
When the ASD is closed, the afterload that the right ventricle has to act against is suddenly increased, and may cause immediate right ventricular failure, since it may not be able to pump the blood against the pulmonary hypertension.
Surgical closure of an ASD involves opening up at least one atrium and closing the defect with a patch under direct visualization.
A percutaneous device closuring involves the passage of a catheter into the heart through the femoral vein guided by fluoroscopy and echocardiography.
A percutaneous device has discs that can expand to a variety of diameters at the end of the catheter.
The catheter is guided through the atrial septal wall and one disc in the left atrium is opened and pulled into place.
Subsequently the other disc in the right atrium is opened in place and the device is inserted into the septal wall.
This type of PFO closure is more effective than drug or other medical therapies for decreasing the risk of future thromboembolism.
PFO device closures most common adverse effect is new-onset atrial fibrillation.
Other complications of device closure are all rare, include device migration, erosion and embolization and device thrombosis or formation of an inflammatory mass with risk for recurrent ischemic stroke.
Percutaneous closure of an ASD is currently only indicated for the closure of secundum ASDs with a sufficient rim of tissue around the septal defect so that the closure device does not impinge upon the superior vena cava, inferior vena cava, or the tricuspid or mitral valves.
The Amplatzer Septal Occluder (ASO) is commonly used to close ASDs, and consists of two self-expandable round discs connected to each other with a 4-mm waist, made up of 0.004– to 0.005-inch Nitinol wire mesh filled with Dacron fabric.
Implantation of the The Amplatzer Septal Occluder device is relatively easy.
The prevalence of residual defect is low after the device placement.
The Amplatzer Septal Occluder device disadvantages are a thick profile of the device and concern related to a large amount of nitinol, a nickel-titanium compound, in the device and consequent potential for nickel toxicity.
Such a percutaneous closure is the method of choice in most centers.
