 Atoms are the basic building blocks of all matter.
Atoms are the basic building blocks of all matter.
Atoms are extremely small, typically around 100 picometers across.
A human hair is about a million carbon atoms wide.
Atoms are the smallest units of matter that retain the properties and characteristics of a particular chemical element.
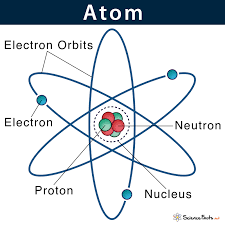
Each atom consists of a central nucleus containing protons and neutrons, which are surrounded by electrons that orbit the nucleus.
The number of protons in the nucleus of an atom determines the element it represents.
This number is called the atomic number and is represented by the symbol “Z”.
The total number of protons and neutrons in the nucleus is called the atomic mass number and is represented by the symbol “A”.
The electrons in an atom are arranged in energy shells or orbitals around the nucleus.
Each shell can hold a specific number of electrons, and the outermost shell is known as the valence shell.
The electrons in the valence shell determine the chemical behavior of the element, including its reactivity and ability to form chemical bonds with other atoms.
Atoms can combine with other atoms to form molecules or compounds, which play a vital role in chemical reactions and the formation of various substances.
Understanding the properties and behavior of atoms is essential inchemistry, physics, and materials science.
