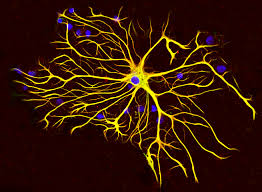
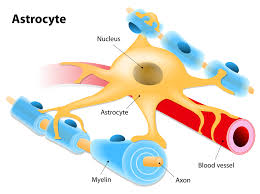
 Astrocytes are a type of glial cell found in the central nervous system.
Astrocytes are a type of glial cell found in the central nervous system.
They are shaped like stars:astrocyte.
They are star-shaped cells that are critical for the normal functioning of the nervous system.
They outnumber neurons in the CNS by about 10 to 1.
These cells have several important functions, including maintaining the chemical balance in the brain, providing for the blood-brain barrier, providing support for neurons, and structural and nutritional support and oxygen, and by helping to maintain the appropriate chemical environment for neuronal function.
Astrocytes also play a role in the formation and maintenance of synapses, that allow for communication in the brain.
Astrocytes play a critical role in regulating the levels of neurotransmitters in the brain: removing excess neurotransmitters from the synapse, and also participate in the reuptake of certain neurotransmitters.
Astrocytes interact with neurons at synapses and play a crucial role in modulating synaptic function.
Astrocytes have a vital role in the normal functioning of the nervous system and are involved in many important processes that are essential for neural activity, blood flow, and injury recovery.
They can alter synaptic strength and plasticity, and can also release their own neurotransmitters to affect or inhibit neuronal activity.
Dysfunction of astrocytes has been implicated in various neurological disorders, such as Alzheimer’s disease and epilepsy.
Astrocytes express glial fibrillary acidic protein (GFAP)
Astrocytes are the most abundant type of macroglial cell in the CNS.
Astrocytes collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord.
Astrocytes are a sub-type of glial cells in the central nervous system, and are also known as astrocytic glial cells.
They are the most abundant glial cells in the brain that are closely associated with neuronal synapses.
They regulate the transmission of electrical impulses within the brain.
Astrocytes have numerous projections that link neurons to their blood supply while forming the blood–brain barrier.
Astrocytes regulate the external chemical environment of neurons by removing excess potassium ions, and recycling neurotransmitters released during synaptic transmission.
Astrocytes may regulate vasoconstriction and vasodilation by producing substances such as arachidonic acid, whose metabolites are vasoactive.
Astrocytes signal each other using ATP.
The gap junctions between astrocytes allow the messenger molecule IP3 to diffuse from one astrocyte to another.
IP3 activates calcium channels on cellular organelles, releasing calcium into the cytoplasm.
This calcium may stimulate the production of more IP3 and cause release of ATP through channels in the membrane made of pannexins.
The net effect is a calcium wave that propagates from cell to cell.
Extracellular release of ATP, and consequent activation of purinergic receptors on other astrocytes, may also mediate calcium waves in some cases.
Astrocytes function include: biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries.
Astrocytes help form the physical structure of the brain, and are thought to play a number of active roles, including the secretion or absorption of neural transmitters and maintenance of the blood–brain barrier.
The astrocyte proportion varies by region and ranges from 20% to around 40% of all glia.
Adtrocytes may be the most numerous cell type in the brain.
Astrocytes are the major source of cholesterol in the central nervous system.
Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain.
Astrocytes propagate intercellular Ca2+ waves over long distances in response to stimulation, and, similar to neurons, release transmitters (gliotransmitters) in a Ca2+-dependent manner, and
signal neurons through Ca2+-dependent release of glutamate.
Astrocyte processes envelop synapses made by neurons.
A single astrocyte cell can interact with up to 2 million synapses at a time.
Several forms of astrocytes exist in the central nervous system: fibrous (in white matter), protoplasmic (in grey matter), and radial.
The fibrous glia are usually located within white matter, have relatively few organelles, and exhibit long unbranched cellular processes.
Fibrous glia often have astrocytic endfoot processes that physically connect the cells to the outside of capillary walls.
Protoplasmic glia are the most prevalent and are found in grey matter tissue, possess a larger quantity of organelles, and exhibit short and highly branched tertiary processes.
The radial glial cells abut the pia mater, while the other is deeply buried in gray matter.
Radial glia are mostly present during development, playing a role in neuron migration.
When in proximity to the pia mater, all three forms of astrocytes send out processes to form the pia-glial membrane.
Astrocytes contain glycogen and are capable of gluconeogenesis.
Astrocytes next to neurons in the frontal cortex and hippocampus store and release glucose.
Astrocytes can fuel neurons with glucose during periods of high rate of glucose consumption and glucose shortage.
Astrocytes provide neurons with nutrients such as lactate,
Glucose sensing is normally associated with neurons, so the detection of interstitial glucose levels within the brain is also controlled by astrocytes.
Astrocytes become activated by low glucose and this activation increases gastric emptying to increase digestion.
Astrocyte activity is linked to blood flow in the brain, and that this is what is actually being measured in fMRI.
Astrocytes express plasma membrane transporters such as glutamate transporters for several neurotransmitters, including glutamate, ATP, and GABA.
Astrocytes express potassium channels at a high density.
When neurons are activated they release potassium, increasing the local extracellular concentration, which is cleared by highly permeable astrocytes.
If the function of clearing potassium by astrocytes is impaired
the extracellular concentration of potassium will rise, leading to neuronal depolarization.
The abnormal accumulation of extracellular potassium is well known to result in epileptic neuronal activity.
Rapid changes in astrocyte morphology have been shown to affect heterosynaptic transmission between neurons.
In the hippocampus, astrocytes suppress synaptic transmission by releasing ATP, which is hydrolyzed by ectonucleotidases to yield adenosine.
Adenosine acts on neuronal adenosine receptors to inhibit synaptic transmission.
Astrocytes may serve as intermediaries in neuronal regulation of blood flow.
Electrical activity in neurons causes them to release ATP, which serves as an important stimulus for myelin to form.
However, the ATP does not act directly on oligodendrocytes.
ATP causes astrocytes to secrete cytokine leukemia inhibitory factor (LIF), a regulatory protein that promotes the myelinating activity of oligodendrocytes: suggesting that astrocytes have an executive-coordinating role in the brain.
Upon injury to nerve cells within the central nervous system, astrocytes fill up the space to form a glial scar, and may contribute to neural repair.
The glial scar may be a barrier to regeneration, thus implicating a negative role in axon regeneration.
However, other studies find astrocytes are required for regeneration to occur.
It is debated whether astrocytes integrate learning and memory in the hippocampus.
Dorsal horn of the spinal cord, activated astrocytes have the ability to respond to almost all neurotransmitters and, upon activation, release a multitude of neuroactive molecules such as glutamate, ATP, nitric oxide (NO), and prostaglandins (PG), which in turn influences neuronal excitability.
Tripartite synapse reflects the close association between astrocytes and presynaptic and postsynaptic terminals as well as their ability to integrate synaptic activity and release neuromodulators.
Astrocytomas are primary intracranial tumors that develop from astrocytes.
These tumors may occur in many parts of the brain and/or spinal cord.
Astrocytomas are divided into two categories: low grade (I and II) and high grade (III and IV).
Low grade astrocytomas tumors are more common in children, and high grade tumors are more common in adults.
Malignant astrocytomas are more prevalent among men, contributing to worse survival.
Pilocytic astrocytomas are grade I tumors.
They are considered benign and slow growing tumors.
Pilocytic astrocytomas frequently have cystic portions filled with fluid and a nodule, which is the solid portion.
Most are located in the cerebellum.
Therefore, most symptoms are related to balance or coordination difficulties.
They also occur more frequently in children and teens.
Fibrillary astrocytomas are grade II tumors.
They grow relatively slowly so are usually considered benign, but they infiltrate the surrounding healthy tissue and can become malignant.
Fibrillary astrocytomas commonly occur in younger people, who often present with seizures.
Anaplastic astrocytomas are grade III malignant tumors.
They grow more rapidly than lower grade tumors.
Anaplastic astrocytomas recur more frequently than lower grade tumors because their tendency to spread into surrounding tissue makes them difficult to completely remove surgically.
Glioblastoma multiforme is a grade IV cancer that may originate from astrocytes or an existing astrocytoma.
Approximately 50% of all brain tumors are glioblastomas.
Glioblastomas can contain multiple glial cell types, including astrocytes and oligodendrocytes.
Glioblastomas are generally considered to be the most invasive type of glial tumor, as they grow rapidly and spread to nearby tissue.
Treatment may be complicated, because one tumor cell type may die off in response to a particular treatment while the other cell types may continue to multiply.
Astrocytes are participants in various neurodevelopmental disorders.
Astrocyte dysfunction may result in improper neural circuitry, which underlies certain psychiatric disorders such as autism spectrum disorders and schizophrenia.
Under normal conditions, pain conduction begins with some noxious signal followed by an action potential carried by nociceptive (pain sensing) afferent neurons, which elicit excitatory postsynaptic potentials in the dorsal horn of the spinal cord.
That message is then relayed to the cerebral cortex, where excitatory postsynaptic potentials translate into pain.
The pain-potentiating synapse located in the dorsal horn of the spinal cord and astrocytes encapsulate these synapses.
There is a correlation between astrocyte hypertrophy in the dorsal horn of the spinal cord and hypersensitivity to pain after peripheral nerve injury, typically considered an indicator of glial activation after injury.
Astrocytes detect neuronal activity and can release chemical transmitters, which in turn control synaptic activity.
Clinically significant pathologies involving astrocytes include astrogliosis and astrocytopathy. multiple sclerosis, anti-AQP4+ neuromyelitis optica, Rasmussen’s encephalitis, Alexander disease, and amyotrophic lateral sclerosis.
Studies have shown that astrocytes may be implied in neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Stuttering and amyotrophic lateral sclerosis, and in acute brain injuries, such as intracerebral hemorrhage and traumatic brain injury.
Gomori-positive Astrocytes and Brain Dysfunction
Astrocytes that possess prominent cytoplasmic granules that are intensely stained by Gomori’s chrome alum hematoxylin stain, and hence are termed Gomori-positive (GP) astrocytes can be found throughout the brain, but are by far the most abundant in the olfactory bulbs, medial habenula, dentate gyrus of the hippocampus, arcuate nucleus of the hypothalamus, and in the dorsal medulla, just beneath the area postrema.
Gomori-positive cytoplasmic granules are derived from damaged mitochondria engulfed within lysosomes.
Cytoplasmic granules contain undigested remnants of mitochondrial structures, including heme-linked copper and iron atoms remaining from mitochondrial enzymes.
Oxidative stress is believed to be cause of damage to these astrocytes.
Brain regions enriched in Gomori-positive (GP) astrocytes also contain a sub-population of specialized astrocytes that synthesize Fatty Acid Binding Protein 7 (FABP7).
Astrocytes, but not neurons, possess the mitochondrial enzymes needed to metabolize fatty acids, and that the resulting oxidative stress can damage mitochondria.
The increased uptake and oxidation of fatty acids in glia containing FABP7 to causes the oxidative stress and damage to mitochondria in these cells.
Astrocytes thus play a key role in lipid synthesis and lipid distribution by releasing lipid carrier proteins, such as Apolipoprotein E, and in maintaining the highway for distribution, the glymphatic system.
FABP proteins interact with a protein called synuclein to cause mitochondrial damage.
Hypothalamic function declines in aging that may be related to GP astrocytes.
GP astrocytes are in close contact with neurons that make the neurotransmitter dopamine in both the hypothalamus.
Dopamine produced by these neurons is carried to the nearby pituitary gland to inhibit the release of a hormone called prolactin from the pituitary.
The activity of dopaminergic neurons declines during aging, leading to elevations in blood levels of prolactin that can provoke breast cancer.
An aging-associated change in astrocyte function might contribute to this change in dopaminergic activity.
FABP7+ astrocytes in close contact with neurons in the arcuate nucleus of the hypothalamus are responsive to leptin produced by fat cells.
Leptin-sensitive neurons in the hypothalamus regulate appetite and body weight.
FABP7+ astrocytes regulate the responsiveness of these neurons to leptin.
Mitochondrial damage in these astrocytes could thus alter the function of leptin-sensitive neurons and could contribute to an aging-associated dysregulation of feeding and body weight.
Gomori-positive (GP astrocytes may also be involved in the hypothalamic regulation of overall glucose metabolism.
Astrocytes function as glucose sensors and exert influence upon neuronal reactivity to changes in extracellular glucose.
GP astrocytes possess high-capacity GLUT2-type glucose transporter proteins and modulate the neuronal responses to glucose.
Hypothalamic astrocytes monitor blood levels of glucose and exert an influence upon blood glucose levels via an altered input to autonomic circuits that innervate liver and muscle cells.
Dysfunction of FABP7+/Gomori-positive astrocytes may contribute to the development of diabetes mellitus.
FABP7+/Gomori-positive astrocytes may play a role in Alzheimer’s disease.
Astrocytes aid in the regulation of neural stem cells.
The brain has many neural stem cells, which are kept in a dormant state by chemical signals from astrocytes.
The astrocytes are able to activate the stem cells to transform into working neurons.
In general, there are two types of astrocytes, protoplasmic and fibrous, similar in function but distinct in morphology and distribution.
Protoplasmic astrocytes have short, thick, highly branched processes and are typically found in gray matter.
Fibrous astrocytes have long, thin, less branched processes and are more commonly found in white matter.
Astrocyte activity is linked to blood flow in the brain, and that this is what is actually being measured in fMRI.
Astrocytes also have been involved in neuronal circuits playing an inhibitory role after sensing changes in extracellular calcium.
