 Aortic dissection (AD) occurs when an injury to the innermost layer of the aorta allows blood to flow between the layers of the aortic wall, forcing the layers apart.
Aortic dissection (AD) occurs when an injury to the innermost layer of the aorta allows blood to flow between the layers of the aortic wall, forcing the layers apart.
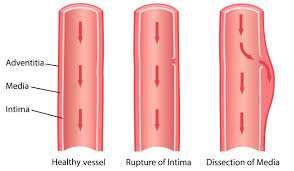
Separation of the layers within the aortic wall.
With aortic dissection blood penetrates the intima and enters the media layer.
The aorta is made up of three layers, the intima, the media, and the adventitia.
The intima is in direct contact with the blood inside the vessel, and mainly consists of a layer of endothelial cells on a basement membrane.
With aortic dissection, blood penetrates the intima and enters the media layer at high pressure that rips the tissue of the media apart along the laminated plane splitting the inner two-thirds and the outer one-third of the media apart.
The initiating event in aortic dissection is a tear in the intimal lining of the aorta.
Due to the high pressures in the aorta, blood enters the media at the point of the tear.
The force of the blood entering the media causes the tear to extend.
The blood travels through the media, creating a false lumen.
Separating the false lumen from the true lumen is a layer of intimal tissue known as the intimal flap.
It can be propagated along the length of the aorta for a variable distance forward or backward.
The media contains connective and muscle tissue.
The aorta is protected on the outside by the adventitia, comprising connective tissue.
In most cases of aortic dissection this is associated with a sudden onset of severe tearing chest or back pain.
Also, vomiting, sweating, and lightheadedness may occur.
Other symptoms may result from decreased blood supply to other organs, such as stroke, lower extremity ischemia, or mesenteric ischemia.
Aortic dissection and rupture are the most morbid consequences of thoracic aortic disease: sudden death with an out of hospital mortality rate of 48.6%.
Aortic dissection can quickly lead to death from insufficient blood flow to the heart or complete rupture of the aorta.
Tears of the intima result in propagation of dissection of blood entering the intima-media space.
The propagation of the dissection may be proximal or distal.
Approximately 2.6-4.5 cases per 100,000 person years.
Aortic dissection and aortic aneurysm mortality has risen over the past decades in many developed countries with the rate of increase ranging from 1.2-24.4 fold.
Aortic aneurysm and dissection are caused by the weakness of the aortic wall due to congenital defect, hypertension, chronic inflammation.
Risk factors:
AD is more common in those with a history of high blood pressure; a number of connective tissue diseases that affect blood vessel wall strength including Marfan syndrome and Ehlers–Danlos syndrome; a bicuspid aortic valve; Turner syndrome; major trauma: smoking and previous heart surgery.
Cocaine use, pregnancy, a thoracic aortic aneurysm, inflammation of arteries, and abnormal lipid levels are also associated with an increased risk.
Ruptured aortic aneurysm and dissection Iare associated with high morbidity and mortality rates.
Most aortic dissection is associated with at least moderate aortic enlargement with 90% 4 cm or greater with many having enlargement of 4.5 cm or greater.
In the absence of dissection, aneurysms of the ascending aorta arevasymptomatic and are usually discovered during thoracic imaging for other indications.
Medical therapy to slow growth and reduce risk of ascending aneurysm is understudied.
Chronic processes such as hypertension, sometimes with atherosclerosis, causes fibrosis and thickening of the intimal layer and degradation and apoptosis of smooth muscle cells in the media.
Above processes cause necrosis and fibrosis of the elastic areas of the aortic wall with stiffness and weakness promoting dissection and rupture of the wall.
Involves weakening of any of the three contiguous aortic tissue layers: the intima, media and adventitia.
Cocaine use is involved in 1.8% of patients with acute aortic dissection.
Weakening of one of the layers leads to a tear in the intima permitting entry of blood btween the intima and the adventitia.
Intraluminal pressure extends the dissection process along the aorta with blood filling the space between the dissected layers and it becomes the false lumen.
Congenital collagen defects such as Marfan syndrome or Ehlers-Danlos syndrome associated with cystic medial degeneration, predisposing patients to both aneurysms and dissection.
Hypertension most common process that leads to dissection of the aorta from high shear stress.
Almost 75% of patients with acute aortic dissection have a history of hypertension.
Iatrogenic causes include cardiac procedures, and more common with comorbid processes are diabetes, chronic hypertension, and atherosclerosis.
About 5% of cases are secondary to iatrogenic reasons.
Three classfications are generally used to define location and extent of aortic involvement:Debakey types, Stanford types and anatomical categories proximal and distal.
Debakey: types I and II involve the ascending aorta as does the Stanford type A and the anatomical category proximal.
Debakey type III involves the descending aorta and is similar to Stanford type B and anatomic category distal.
Most intimal tears occur in the ascending aorta.
International Registry of Acute Aortic Dissection findings indicate predisposing factors for acute aortic dissection are hypertension (72%) and atherosclerosis (31%).
International Registry of Acute Aortic Dissection findings indicate that hypertension and atherosclerosis are less important factors for patients under the age of 40 years with only 34% with hypertension and 1% with atherosclerosis.
International Registry of Acute Aortic Dissection analysis includes preexisting factors to be preexisting aortic aneurysm, vasculitis, bicuspid aortic valve, coronary artery bypass, cardiac catheterization, aortic valve replacement, Turner syndrome collagen disease and trauma.
Most type A acute aortic dissection are due to cardiac surgical procedures and most iatrogenic type B acute aortic dissections are due to percutaneous cardiac procedures.
Prognosis:
Mortality without treatment 10% (type B), 50% (type A)
The two main types are Stanford type A, which involves the first part of the aorta, and type B, which does not.
Type A aortic dissection has a mortality rate of 57% without emergency surgery and up to 25% with emergency surgery.
Centers with high case volume have improved outcomes for patients with type A thoracic aortic aneurysms with a mortality rate is 4.4%.
Prevention is by blood pressure control and smoking cessation.
Dissections that involve the first part of the aorta, adjacent to the heart usually require surgery.
Surgery may be done either by an opening in the chest or from inside the blood vessel.
Dissections involving the second part of the aorta can typically be treated with medications that lower blood pressure and heart rate.
If there are complications surgical correction may be required.
AD is relatively rare, occurring at an estimated rate of three per 100,000 people per year.
It is more common in men than women.
The typical age at diagnosis is 63, with about 10% of cases occurring before the age of 40.
Without treatment, about half of people with Stanford type A dissections die within three days and about 10% of people with Stanford type B dissections die within one month.
About 96% of patients with AD have severe pain of sudden onset.
The pain may be described as a tearing, stabbing, or sharp sensation in the chest, back, or abdomen.
About 17% of individuals feel the pain migrate as the dissection extends down the aorta.
The location of pain is associated with the location of the dissection.
Anterior chest pain is associated with dissections involving the ascending aorta, while interscapular back pain is associated with descending aortic dissections.
If the pain is pleuritic in nature, it may suggest acute pericarditis caused by bleeding into the pericardial sac.
Pericardial tamponade is the most common cause of death in aortic dissection.
AD is usually not associated with the other suggestive signs, such as heart failure and ECG changes of myocardial infarction.
Less common symptoms in the setting of AD include congestive heart failure (7%), fainting (9%), stroke (6%), ischemic peripheral neuropathy, paraplegia, and cardiac arrest.
Neurological complications of aortic dissection, such as stroke and paralysis, are due to the involvement of one or more arteries supplying portions of the central nervous system.
If the AD involves the abdominal aorta, compromise of one or both renal arteries occurs in 5–8% of cases, while ischemia of the intestines occurs about 3% of the time.
People with AD often have a history of hypertension.
The blood pressure tends be higher in individuals with a distal dissection.
In individuals with a proximal AD, 36% present with hypertension, while 25% present with hypotension.
Proximal AD tends to be associated with weakening of the vascular wall due to cystic medial degeneration.
In those who present with distal (Stanford type B) AD, 60–70% present with high blood pressure, while 2–3% present with low blood pressure.
Severe hypotension at presentation is a grave prognostic indicator.
Severe hypotension with AD is usually associated with pericardial tamponade, severe aortic insufficiency, or rupture of the aorta.
Pseudohypotension may occur due to involvement of the brachiocephalic artery or the left subclavian artery.
Aortic insufficiency occurs in half to two-thirds of ascending AD, and the diastolic heart murmur of aortic insufficiency is audible in about 32% of proximal dissections.
Aortic insufficiency may occur with AD with dilation the annulus of the aortic valve, preventing the leaflets of the valve from coapting.
Dissection may extend into the aortic root and detach the aortic valve leaflets.
An extensive intimal tear may prolapse into the left ventricular outflow tract, causing intimal intussusception into the aortic valve, thereby preventing proper valve closure.
Heart attack occurs in 1–2% of aortic dissections, caused by the involvement of the coronary arteries.
The right coronary artery is involved more commonly than the left coronary artery.
If the myocardial infarction is treated with thrombolytic therapy, the mortality increases to over 70%, mostly due to bleeding into the pericardial sac, causing cardiac tamponade.
If a pleural effusion were to develop due to AD, it is more common in the left hemithorax rather than the right hemithorax.
AD is associated with hypertension and many connective tissue disorders.
Vasculitis is rarely associated with aortic dissection.
IAD can also be the result of chest trauma.
About 72 to 80% of individuals who present with an aortic dissection have a previous history of hypertension.
Illicit drug use with stimulants such as cocaine and methamphetamine is also a modifiable risk factor.
It can also be caused by smoking.
A bicuspid aortic valve is found in 7–14% of individuals who have an aortic dissection.
These individuals are prone to dissection in the ascending aorta.
Connective tissue disorders increase the risk of aortic dissection:
Marfan syndrome, Ehlers–Danlos syndrome, and Loeys–Dietz syndrome.
Vasculitides such as Takayasu’s arteritis, giant cell arteritis, polyarteritis nodosa, and Behçet’s disease have been associated with aortic dissection.
Marfan Syndrome accounts for 5–9% of individuals who had an aortic dissection.
Individuals with Marfan syndrome tend to be young and have aneurysms of the aorta and are more prone to proximal dissections of the aorta.
Turner syndrome also increases the risk of aortic dissection, by aortic root dilatation.
Chest trauma leading to aortic dissection: blunt chest trauma and iatrogenic.
Iatrogenic causes include trauma during cardiac catheterization or due to an intra-aortic balloon pump.
About 18% of individuals who present with an acute aortic dissection have a history of open-heart surgery.
Patients who have undergone aortic valve replacement for aortic insufficiency are at particularly high risk because aortic regurgitation causes increased blood flow in the ascending aorta, causing dilatation and weakening of the walls of the ascending aorta.
Syphilis potentially causes aortic dissection in its tertiary stage.
Dissections that propagate towards the iliac bifurcation are called anterograde dissections.
Dissections that propagate towards the aortic root, which is opposite of the flow of blood, are called retrograde dissections.
If the aortic initial tear is within 100 mm of the aortic valve,a retrograde dissection can easily compromise the pericardium leading to a hemopericardium.
Anterograde dissections may propagate to the iliac bifurcation of the aorta, rupture the aortic wall, or recanalize into the intravascular lumen leading to a double-barrel aorta.
The presence of a double-barreled aorta relieves the pressure of blood flow and reduces the risk of rupture.
Rupture leads to hemorrhaging into a body cavity: Retroperitoneal and pericardial ruptures are both possible.
The vast majority of aortic dissections originate with an intimal tear in either the ascending aorta (65%), the aortic arch (10%), or just distal to the ligamentum arteriosum in the descending thoracic aorta (20%).
As blood flows down the false lumen, it may cause secondary tears in the intima, and through these secondary tears, the blood can re-enter the true lumen.
The intimal tear may occur from degeneration of the collagen and elastin that make up the media, and this is known as cystic medial necrosis.
Cystic medial necrosis is most commonly associated with Marfan syndrome and is also associated with Ehlers-Danlos syndrome.
In about 13% of aortic dissections, no evidence of an intimal tear is found. In these cases, and the inciting event is bleeding within the media.
Diagnosis:
The diagnosis is sometimes difficult to make, as it cannot always be made by history and physical signs alone.
Common testing used to diagnose an aortic dissection include: a CT scan of the chest with iodinated contrast material and a transesophageal echocardiogram.
High frequency ultrasound, aortogram or magnetic resonance angiogram of the aorta may be used.
A measurement of blood D-dimer level may be useful in diagnostic evaluation:A level less than 500 ng/ml may be considered evidence against a diagnosis of aortic dissection.
Evidence of value of D-dimmer test is tentative.
Chest X-Ray may a demonstrate a change in the shape of the thoracic aorta which can be seen in aortic dissection.
A new widening of the mediastinum on radiograph is of moderate sensitivity for detecting an ascending aortic dissection, is of low specificity, as many other conditions can cause apparent widening of the mediastinum.
Other findings: calcium sign-describes an apparent separation of the intimal calcification from the outer aortic margin by greater than 10 mm.
Pleural effusions, more commonly in descending aortic dissections, and typically left-sided.
Obliteration of the aortic knob,
Depression of the left mainstem bronchus, loss of the paratracheal stripe, and tracheal deviation.
About 12 to 20% of aortic dissections are not detectable by chest radiograph: a normal chest x-ray does not rule out an aortic dissection.
Computed tomography angiography gives an accurate three-dimensional view of the aorta.
To define the aorta to make the proper diagnosis, an iodinated contrast material is injected into a peripheral vein.
A scan is timed to injection to capture the contrast as it enters the aorta.
A CT angiographic scan has a sensitivity of 96 to 100% and a specificity of 96 to 100%.
Disadvantages include the need for iodinated contrast material and the inability to diagnose the site of the intimal tear.
CT with contrast can demonstrate an aneurysmal dilation and a dissection of the ascending and descending aorta .
Magnetic resonance imaging (MRI) is also used for the detection and assessment of aortic dissection, and has a sensitivity of 98% and a specificity of 98%.
An MRI examination of the aorta produces a three-dimensional view allowing the determination of the location of the intimal tear and the involvement of branch vessels, and to locate any secondary tears.
MRI is a noninvasive test, does not require the use of iodinated contrast material, and can detect and quantitate the degree of aortic insufficiency.
A transesophageal echocardiogram (TEE) has a sensitivity up to 98% and a specificity up to 97% for the diagnosis of aortic dissection, and
has become the preferred imaging modality for suspected aortic dissection.
Transesophageal echocardiogram can evaluate aortic insufficiency in the setting of ascending aortic dissection and to determine whether the ostia of the coronary arteries are involved.
Disadvantages of TEE include the inability to visualize the distal ascending aorta, and the descending abdominal aorta that lies below the stomach.
An aortogram involves the placement of a catheter in the aorta and injection of contrast material while taking X-rays of the aorta: it has been replaced by other, less-invasive imaging modalities.
Several different classification systems describe aortic dissections.
One such classification labels aortic dissections as hyperacute (<24 hours duration), acute (2–7 days), subacute (8–30 days), and chronic (>30 days).
Other systems in use are based on either the anatomy of the dissection or the duration of onset of symptoms before the presentation.
The DeBakey system is an anatomical description of the aortic dissection, based on where the original intimal tear is located and the extent of the dissection.
Type I – originates in ascending aorta.
It propagates at least to the aortic arch and often beyond it distally.
This type is most often seen in patients less than 65 years of age and is the most lethal form of the disease.
Type II – originates in the ascending aorta and is confined to it.
Type III – originates in the descending aorta and rarely extends proximally, but will extend distally.
Type III AD most often occurs in elderly patients with atherosclerosis and hypertension.
The Stanford aortic dissection classification is divided into two groups, A and B, depending on whether the ascending aorta is involved.
A – involves the ascending aorta and/or aortic arch, and possibly the descending aorta.
The tear can originate in the ascending aorta, the aortic arch, or more rarely, in the descending aorta: includes DeBakey types I and II.
B – involves the descending aorta or the arch distal to the left subclavian artery, without the involvement of the ascending aorta:
It includes DeBakey type III.
The Stanford type A ascending aortic dissections generally require primary surgical treatment, whereas type B dissections generally are treated medically as initial treatment with surgery reserved for any complications.
The main indication for surgical repair of type A dissections is the prevention of acute hemorrhagic pericardial tamponade from leakage of blood through the dissected layers of the intrapericardial proximal aorta.
A secondary indication is acute aortic valve insufficienc.
Ascending aortic dissections often involve the aortic valve, which, telescopes down into the aortic root, resulting in aortic incompetence.
The valve must be reseated, as well as to repair or prevent coronary artery injury.
Also, the area of dissection is removed and replaced with a Dacron graft to prevent further dissection from occurring.
Type B dissections are not improved, from a mortality point of view, unless leaking, rupture, or compromise to other organs, e.g. kidneys, occurs.
Prevention
Hypertension, and high levels of lipids in the blood, and smoking tobacco are considered preventable risk factors.
Repair of an enlargement of the ascending aorta from an aneurysm or previously unrecognized and untreated aortic dissections is recommended when greater than 5.5 cm in size to decrease the risk of dissection.
Repair may be recommended when greater than 4.5 cm (1.8 in) in size if the person has one of the several connective-tissue disorders or a family history of a ruptured aorta.
For Stanford type A, ascending, aortic dissection, surgical management is superior to medical management.
For uncomplicated Stanford type B, distal aortic, dissections, including abdominal aortic dissections, medical management is preferred over surgery.
Complicated Stanford type B aortic dissections require surgical intervention .
The risk of death due to aortic dissection is highest in the first few hours after the dissection.
An acute dissection: the individual presents within the first two weeks.
These who survive to. 2 weeks have improved prognosis.
About 2/3 of all dissections present in the acute phase.
Individuals who present two weeks after the onset of the dissection have chronic aortic dissections, and can be treated with medical therapy as long as they are stable.
Management:
Aortic dissection generally presents as a hypertensive emergency, and the prime consideration of medical management is to decrease the shear stress in the aortic wall by decreasing blood pressure and the heart rate.
The target blood pressure should be a mean arterial pressure (MAP) of 60 to 75 mmHg, or the lowest blood pressure tolerated.
Initial BP decreases should be by about 20%.
The target heart rate is less than 65 beats per minute.
Long-term blood pressure control is required.
THE first-line treatment for patients with acute and chronic aortic dissection are beta-blocker agents.
In acute dissection, fast-acting agents can be given intravenously are preferred.
Vasodilators such as sodium nitroprusside can be considered for people with ongoing high blood pressure, but they should never be used alone, as they often stimulate a reflexive increase in the heart rate.
Calcium channel blockers can be used in the treatment of aortic dissection.
The calcium channel blockers typically used are verapamil and diltiazem, because of their combined vasodilator and negative inotropic effects.
Indications for the surgical treatment of aortic dissection include an acute proximal aortic dissection and an acute distal aortic dissection with one or more complications.
Complications include: compromise of a vital organ, rupture or impending rupture of the aorta, retrograde dissection into the ascending aorta.
Aortic dissection complications are more common with a history of Marfan syndrome or Ehlers-Danlos syndrome.
Surgical management of aortic dissection is to resect the damaged segments of the aorta and to obliterate the entry of blood into the false lumen.
Thoracic endovascular aortic repair, a minimally invasive surgical procedure usually combined with ongoing medical management
Comorbid conditions increase the surgical risk of repair of an aortic dissection:
Prolonged preoperative evaluation
Advanced age
Comorbid disease states
Aneurysm leakage
Cardiac tamponade
Shock – obstructive shock
Past history of myocardial infarction
History of kidney failure (either acute or chronic kidney failure)
The risk of late rupture of an aortic aneurysm is 10 times higher in individuals who have uncontrolled hypertension, compared to individuals with a systolic pressure below 130 mmHg.
The risk of death is highest in the first two years after the acute aortic dissection.
About 29% of late deaths following surgery are due to rupture of either a dissecting aneurysm or another aneurysm.
In addition, a 17% to 25% incidence exists of new aneurysm formation, typically due to dilatation of the residual false lumen.
Risk of death in untreated type A aortic dissection
25% in first 24 hours
50% in first 72 hours
80% in two weeks
90% in first month
Of all people with aortic dissection, 40% die immediately and do not reach a hospital in time.
Of the remainder, 1% die every hour, making prompt diagnosis and treatment a priority.
Even after diagnosis, 5–20% die during surgery or in the immediate postoperative period.
In ascending aortic dissection, if surgery is decided to be not appropriate, 75% die within 2 weeks.
With aggressive treatment, 30-day survival for thoracic dissections may be as high as 90%.
Aortic dissection affects an estimated 2.0–3.5 people per 100,000 each year.
The incidence of aortic dissection may be rising.
65% of all people with aortic dissection are male.
The mean age at diagnosis is 63 years.
In females before the age of 40, half of all aortic dissections occur during pregnancy.
Aortic dissection occurs in about 0.6% of pregnancies.
Most iatrogenic acute aortic dissections do not present with typical symptoms and imaging studies do not show classic findings.
Chest pain is the most common symptom on presentation occurring in 72.7% patients (International Registry of Acute Aortic Dissection [IRAD]).
Other presenting symptoms include cerebrovascular insufficiency/accident, heart failure, syncope, pulse deficit, aortic regurgitation and shock.
Painless aortic dissection is a chance for 6.4% of presentations and is associated with the less favorable outcome then painful presentations(Park SW).
Aortic tears can extend into a false lumen, and can occlude blood flow from the lumen of the aorta into arteries that originate from it.
Occlusion of arteries from aortic dissection can cause a variety of symptoms including a new myocardial infarction, syncope, hemiplegia, flank pain, paraplegia or quadraplgia or death.
Can cause myocardial infarction with ST segment elevation when the dissection propagates approximately 2 involve a coronary artery, which most commonly is the right coronary artery.
ST-segment elevation may occur because of obstruction of the ostium of the right coronary artery.
Of 464 patients with aortic dissection 4.8% involving the ascending aorta had new ST segment changes or Q waves consistent with acute myocardial infarction (Hagan PG et al).
Hemopericardium may be present and lead to tamponade.
Transesophageal echocardiography has high sensitivity and specificity for this diagnosis.
Initial management involves lowering the blood pressure and decreasing the velocity of the left ventricle contraction.
By decreasing the aortic shear stress one can minimize the propagation of the dissection.
Beta blockers are the initial treatment of choice because they can decrease the volume of contraction and systemic blood pressure.
Nitroprusside can be used as it is an arterial and venous dilator but should be utilized with Beta blockers because vasodilation alone may induce reflex activation of the sympathetic nervous system that will enhance ventricular contraction and increase aortic shear stress.
Patients with iatrogenic dissections are often older than typical patients and have a higher incidence of vascular risk factors.
Many patients die before presentation or before diagnosis is made.
One quarter to one half of aortic dissections in women under age 40 occurs during pregnancy, typically in the third trimester or early postpartum period (O’Gara PT).
Hormones of pregnancy may alter the integrity of elastin fibers with predisposition to dissection.
Diagnosis is primarily achieved by history and physical exam.
Chest x-rays, classically show widened mediastinum.
IRAD data reveal no abnormal mediastinal or abnormal aortic contours in 21.3% of patients.
Thoracic CT scans and transesophageal echocardiogram imaging are techniques of choice for diagnosis, as both are associated with a sensitivity and specificity of greater than 90%.
CT of the thorax is the first consideration in the evaluation of the suspected aortic dissection because of its accuracy, safety, speed, and availability.
If not treated mortality rate during the first 24-48 hours is as high as 1-2% per hour.
More common in males than females, with a 2:1 ratio.
Most common presentation in the sixth and seventh decades.
Patients with Marfan’s syndrome usually present in the third and fourth decades.
The partition of the media of the aorta is frequently horizontal or diagonal.
The intima tear connects the media with the lumen of the aorta and an exit tear and creates a true lumen or a false lumen.
The true lumen is lined by intima and the false lumen is within the media.
Blood flow in a false lumen is usually slower than in a true lumen.
The false lumen can become an aneurysm when subject to systemic pressure.
Dissection usually stops at an aortic branch or at the site of an atheroslerotic plaque.
The vast number of cases associated with atherosclerosis occur in the ascending aorta while the severity of this process is less severe in the descending aorta.
When a type A dissection is identified by CT scan an emergent transesophageal echocardiogram can be performed to evaluate anatomy and aortic valve competence and then can proceed to emergent surgery.
Patients with type B aortic dissections that are uncomplicated may be managed medically.
Type B aortic dissections are associated with a 10% in hospital mortality with medical management and a 60-80% five-year survival rate.
Type A dissections require emergency surgery to avoid intra-pericardial rupture, cardiac tamponade or myocardial infarction.
If left untreated type A aortic dissections have a mortality rate as high as 1-2% per hour after symptom onset if surgically untreated (Niena Butber CA et al).
Surgery for aortic dissection aims to dissect the damaged aorta, excise the intimal tear, and a obliterate the entry into the false lumen.
Surgery is performed via a median sternotomy and involves dissection from inside the aorta to access the intima and media damage and to remove the damaged segments.
A Dacron prosthetic sleeve graft is utilized to rebuild the aorta and reestablish vascular continuity between the ends of the aorta.
Use of fluoroquinolones associated with an increased risk of aortic aneurysm and dissection.

One reply on “Aortic dissection”
Atherosclerosis of the aorta is a serious health condition that can lead to various complications, including AAA, aortic dissection, and PAD. This condition occurs when plaques made of cholesterol and other substances build up on the inner walls of the aorta, narrowing and hardening the artery over time. Check out this article for more information. best doctor for atherosclerosis of aorta