 Also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat.
Also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat.
Under the influence of energy stress adipocytes may degrade their stored fat to supply fatty acids and also glycerol to the circulation.
Like no other cells, adipocytes are adept at enlarging and can swell from a diameter of 30 to 40 mm to more than 100 mm, an increase in volume by a factor of more than 10.
Starting in early adulthood, lean adults gain an average 0.5 kg per year of adipose tissue, and with even higher weight increases among the overweight and obese, put an enormous demand on white adipose depots to store triglycerides by the fifth and six decades of life
The metabolic activities of adipocytes are regulated by several hormones: insulin, glucagon and epinephrine.
Changes in adipocyte function affect systemic metabolism, including insulin sensitivity in the liver and muscle, insulin secretion from pancreatic beta cells, and food intake and energy expenditure, regulated by the brain and sympathetic nervous system, ultimately affecting body weight.
Adipose tissue is an endocrine gland that releases hormones in the bioactive molecules referred to as adipokines.
Adipose tissue also secretes the hormone leptin.
There are two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), which are also known as white fat and brown fat, respectively, and comprise two types of fat cells.
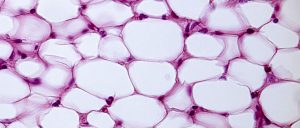
White fat cells or monovacuolar cells contain a large lipid droplet surrounded by a layer of cytoplasm, and the nucleus is flattened and located on the periphery.
A typical fat cell is 0.1 mm in diameter.
Fat is stored in a semi-liquid state, and is composed primarily of triglycerides and cholesteryl ester.
White fat cells secrete many proteins acting as adipokines such as resistin, adiponectin, leptin and apelin.
An average human adult has 30 billion fat cells with a weight of 30 lbs or 13.5 kg.
If excess weight is gained as an adult, fat cells increase in size about fourfold before dividing and increasing the absolute number of fat cells present.
Brown fat cells or plurivacuolar cells are polygonal in shape.
Brown fat cells have considerable cytoplasm, with lipid droplets scattered throughout.
In brown fat cells the nucleus is round, but not in the periphery of the cell.
The brown color comes from the large quantity of mitochondria, and is known as “baby fat,” and used to generate heat.
Pre-adipocytes are undifferentiated fibroblasts that can be stimulated to form adipocytes.
The lineage of adipocyte is still unclear, as the variation of body fat distribution resulting from normal growth is influenced by nutritional and hormonal status in dependence on intrinsic differences in cells found in each adipose depot.
Mesenchymal stem cells can differentiate into adipocytes, connective tissue, muscle or bone.
Adipose stromal cells have the power to infiltrate cancers and promote the growth rate of tumors.
Areolar connective tissue is composed of adipocytes.
The term lipoblast is used to describe the precursor of the adult cell.
Even after marked weight loss, the body never loses adipocytes.
With changes in weight, the adipocytes in the body merely gain or lose fat content.
If the adipocytes in the body reach their maximum capacity of fat, they may replicate to allow additional fat storage.
The number of adipocytes can increase in childhood and adolescence, though the amount is usually constant in adults.
A fat cell lives for about 7 years
Individuals who become obese as adults, rather than as adolescents, have no more adipocytes than they had before.
Childhood obesity associated with an inflated number of fat cells.
People who become fat as adults may have no more fat cells than their lean peers, but their fat cells are larger.
Patients with an excess of fat cells find it more difficult to lose weight and keep it off, than the obese who simply have enlarged fat cells.
Body fat cells could have regional responses to overfeeding.
Approximately 10% of fat cells are renewed annually.
Renewal of fat cells at all adult ages and levels of body mass index occurs without a significant increase in the overall number of adipocytes in adulthood.
Obesity is characterized by the expansion of fat mass, through adipocyte size increase and, to a lesser extent, cell proliferation.
In the fat cells of obese individuals, there is increased production of metabolism modulators, such as glycerol, hormones, and pro-inflammatory cytokines, leading to the development of insulin resistance.
Fat production in adipocytes is strongly stimulated by insulin.
Insulin promotes unsaturated fatty acid synthesis, promotes glucose uptake and activates the transcription of genes that stimulate lipogenesis.
Insulin resistance is usually associated with obesity, the membrane phospholipids of the adipocytes of obese patients generally still show an increased degree of fatty acid unsaturation.
Adipocytes can synthesize estrogens from androgens, suggesting the reason being underweight or overweight are risk factors for infertility.
Adipocytes are responsible for the production of the hormone leptin, important in regulation of appetite and acts as a satiety factor.
Adipocytes can synthesize aldosterone directly or by secreting leptin, which stimulates the production of aldosterone by the adrenal gland.
Maturing adipocytes shed nephrilydin from their surfaces and contributes to depression of circulating levels of natriuretic peptide, and in turn increases aldosterone production.
Anatomical location and genetics largely determine the proportional increase in adipocyte size and number and the physiologic responses.
For people with subcutaneous depots maintaining small adipocytes have fewer metabolic complications.
People who are genetically predisposed to impaired catecholamine mediated lipolysis, white adipose adipocytes become very large, a situation exacerbated by genetic inability to recruit new adipocytes.
