 Trypanosoma cruzi is a species of parasitic euglenoids.
Trypanosoma cruzi is a species of parasitic euglenoids.
A protozoa, that characteristically bore tissue in another organism and feed on blood and also lymph.
Over 130 species can transmit this parasite.
Six taxonomic subunits are recognized.
It causes disease Chagas disease in humans.
Parasites need a host body and the haematophagous insect triatomine is the major vector in accord with a mechanism of infection.
The triatomine situate at nests of vertebrate animals for shelter, where it bites and sucks blood for food.
Triatomines infected with protozoa transmit trypanosomes when the triatomine deposits its faeces on the host’s skin surface and then bites.
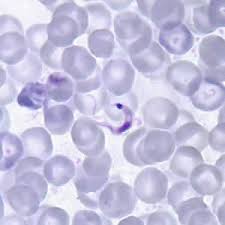
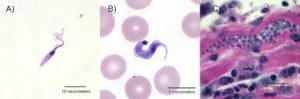
Trypanosoma cruzi in blood are typically seen as a C-shape and have a more pronounced kinetoplast compared to other species.
The Trypanosoma cruzi life cycle starts in an animal reservoir, usually mammals, including humans.
A triatomine bug serves as the vector taking a blood meal, while ingests T. cruzi.
When the triatomine bug subsequently defecates, its waste containing T. cruzi propagation stages.
Alternatively, infection occurs through the oral ingestion of parasites.
It can be transmitted through a number of ways: congenital transmission, blood transfusion, organ transplantation, consumption of uncooked food that has been contaminated with feces from infected bugs, and accidental laboratory exposure.
The trypomastigotes are in the feces and are capable of swimming into the host’s cells using flagella, a characteristic swimming tail.
The trypomastigotes enter the host through the bite wound or by crossing mucous membranes.
As the infection progresses, the number of infected cells increases, as well as the number of amastigotes per infected cell.
The acute form of trypanosomiasis is usually unnoticed, and has elevated parasitism, myocarditis, and changes in the myocardial gene expression.
The chronic form may develop 30 to 40 years after infection and affect internal organs of the heart, esophagus, the colon, and the peripheral nervous system.
Affected people may die from heart failure and severe heart lesions.
The initial response to Trypanosoma cruzi infection is inflammation, and cellular damage occurring in heart tissue, followed by fibrosis.
Nearly all cases of chronic Chagas’ disease experience thromboembolic syndrome.
Trypanosoma cruzi occurrence contributes to the death of a patient by four means: arrhythmias, stasis secondary to cardiac dilation, mural endocarditis, and cardiac fibrosis.
These thrombi also affect other organs such as the brain, spleen and kidney.
Conduction abnormalities are also associated with T. cruzi. due to
abnormalities is a depopulation of parasympathetic neuronal endings on the heart.
The loss of parasympathetic innervations can lead to sudden death due to a severe cardiac failure that occurs during the acute stage of infection.
Chronic Chagas’ disease causes a change in ventricular repolarization, which is represented on an electrocardiogram as the T-wave, and
inhibits the heart from relaxing and properly entering diastole.
Changes in the ventricular repolarization in Chagas’ disease are likely due to myocardial ischemia, and also can lead to fibrillation.
Warming trends may allow vector species to move north. U.S. domestic and wild animals are reservoirs for T. cruzi.
High-risk individuals include those who don’t have access to proper housing.
The incubation period is five to fourteen days after a host comes in contact with feces.
Chagas disease undergoes two phases: acute and the chronic.
The acute phase can last from two weeks to two months.
The acute phase can go unnoticed because symptoms are minor and short-lived.
Symptoms of the acute phase include swelling, fever, fatigue, and diarrhea.
The chronic phase causes digestive problems, constipation, heart failure, and pain in the abdomen.
Diagnostic methods include microscopic examination, serology, or the isolation of the parasite by inoculating blood into a guinea pig, mouse, or rat.
No vaccines are available.
Disease prevention resides within vector control, by the use of insecticides and taking preventative measures such as applying bug repellent on the skin, wearing protective clothing, and staying in higher quality hotels when traveling.
Genetic exchange has been identified among field populations of T. cruzi.[26] This process appears to involve genetic recombination as well as a meiotic mechanism.
