 Tertiary lymphoid organs (TLOs) are structures that develop at specific sites in various tissues in response to chronic inflammation or infection.
Tertiary lymphoid organs (TLOs) are structures that develop at specific sites in various tissues in response to chronic inflammation or infection.
Primary lymphoid organs like the thymus and bone marrow are sites where lymphocytes mature.
Secondary lymphoid organs like lymph nodes and spleen , are where immune responses are initiated.
TLOs form in non-lymphoid tissues, and are characterized by the presence of lymphoid aggregates containing B cells, T cells, and other immune cells.
They play a significant role in local immune responses by facilitating interactions between immune cells, promoting antigen presentation, and generating adaptive immune responses.
TLOs are often found in tissues affected by chronic inflammation, autoimmune diseases, and cancer.
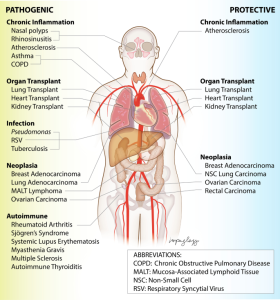
Tertiary lymphoid structures are found in the lungs of individuals with chronic obstructive pulmonary disease (COPD), in the synovial tissues of patients with rheumatoid arthritis, and in tumor tissues during cancer progression.
While TLOs are not anatomically organized in the same way and may lack the distinct regions found in lymph nodes, such as germinal centers.
TLOs can ssupport the generation of antigen-specific immune responses and contribute to local immune surveillance and inflammation control.
Tertiary lymphoid organs (TLOs)are found at sites of chronic inflammation in autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis.
These organized accumulations of T and B cells resemble secondary lymphoid organs and generate autoreactive effector cells.
Autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis are marked by chronic inflammation in end organs that can be associated with the development of tertiary lymphoid organs (TLOs).
TLOs are accumulations of lymphocytes and stromal cells in an organized structure that occur outside of secondary lymphoid organs.
TLOs share many features with secondary lymphoid organs, such as the presence of T and B cell compartmentalization into T cell zones and B cell follicles, chemokines that mediate the compartmentalization, antigen-presenting cells, lymphatic sinuses, high endothelial venules, follicular dendritic cells, and fibroblastic reticular cells (FRCs).
In SLE, there is inflammation of the kidney interstitial tissue, which is associated with greater risk for kidney failure.
In almost 50% of SLE patients patients have well-circumscribed aggregates of B cells, plasma cells, and T cells in he kidney interstitium and a small fraction can have well-organized germinal centers with follicular dendritic cells.
In rheumatoid arthritis, TLOs ranging from B and T cell aggregates to germinal centers are found in the inflamed synovium of about half of patients.
In RA patients such lymphoid aggregates are associated with more severe joint and systemic inflammation.
TLOs are also found in other organs in other autoimmune diseases: salivary and lacrimal glands in Sjögren’s syndrome, the central nervous system in multiple sclerosis, the pancreas in diabetes, the thymus in myasthenia gravis, and the intestines in inflammatory bowel disease.
It is not known whether TLOs provide pathogenic or protective contributions to SLE, rheumatoid arthritis, and other autoimmune diseases.
Tertiary lymphoid organs can generate potentially pathogenic cells.
TLOs have been generally considered to be protective with infections, adopting secondary lymphoid organ-like functions and acting directly as positioned at the site of inflammation.
TLOs can generate effector cells that provide effective host defense.
Innate lymphoid cells (ILCs) in the lung are induced after influenza infection and have been shown to maintain lung function, epithelial integrity, and airway remodeling.
ILCs have been shown to be associated with TLOs and have even been associated with decreased disease progression in lung cancer.
Induced lung TLOs populate and function in a protective manner: influenza virus infection, pulmonary Mycobacterium tuberculosis (MTB) infection 38– 40.
Latent tuberculosis is associated with more frequent, well-organized TLOs.
TLOs are less frequent and less well-formed in active tuberculosis.
These findings suggest a protective role for TLOs in controlling disease.
The TLOs contribute to the formation of granulomas, which
function to promote immunity and limit tissue damage.
CXCL13 expression that organizes the B cell follicles serves to recruit CXCR5-expressing T helper (Th) cells into granulomas to activate macrophages that are essential to infection control.
TLOs to support immune responses that are capable of protecting the host, and similar to immune responses generated in SLOs, immune responses targeted to self may be harmful to the host.
The TLOs correlate with the presence of immune complexes, suggesting that the locally generated antibodies are autoantibodies to renal antigens that can fix complement and thus cause tissue inflammation and damage.
B cell responses associated with TLOs in the rheumatoid arthritis synovium, the salivary glands in Sjögren’s syndrome and other target show autoimmunity.
SLE kidneys and rheumatoid synovium are have an accumulation of Th17 cells, which can have proinflammatory roles.
B cells are necessary for the accumulation of activated T cells, likely by presenting antigen to the T cells and B cells in TLOs may be pathogenic in part by stimulating autoreactive T cells, which then can contribute to the inflammation in the affected end organs.
TLOs can be a source of potentially pathogenic lymphocytes in autoimmunity.
TLOs could also sequester pathogenic lymphocytes and prevent them from leaving the specific tissue or tissue compartment to cause further damage.
TLOs can be protective, providing a microenvironment that generates regulatory or reparative cells that reduce the pathogenicity of inflammatory cells.
In autoimmune diseases, TLOs can generate and harbor autoreactive and proinflammatory, potentially pathogenic lymphocytes but could potentially serve to limit pathogenic responses by sequestering these cells or by reducing the magnitude of the response.
