 Utilized to control back pain when conservative treatment is not successful, when surgery is not a consideration to control pain, when no contraindications are present for its placement, and a trial of the stimulator has been successful.
Utilized to control back pain when conservative treatment is not successful, when surgery is not a consideration to control pain, when no contraindications are present for its placement, and a trial of the stimulator has been successful.
The use of spinal cord stimulation for chronic pain has increased, but evidence for its efficacy and cost effectiveness is limited.
It is estimated up to 50,000 patients currently undergo spinal cord stimulation treatment per year worldwide.
Also known as a dorsal column stimulator.
Two systems for spinal cord stimulation.
Most commonly used is a fully implanted unit utilizing a pulse generator and a non-rechargeable battery that needs replacement over time.
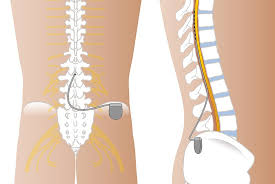
Spinal cord stimulation involves the placement of a subcutaneous implanted pulse generator connected to electrodes with leads that travel into the epidural space posterior to the spinal cord dorsal columns.
Spinal cord stimulation potentially inhibits pain is related to spinal gate control, alteration of neural transmitter levels, promotion of inhibitory interneuron activation, effects on glial and immune cells, and supraspinal mechanisms.
Traditionally spinal cord stimulation management replaces pain with paresthesias.
Spinal cord first stimulation involves intermittent closely spaced, high frequency electrical stimuli of the spinal cord.
Burst spinal cord stimulation may improve the tolerability of intervention offering relief of pain, allowing paresthesia free treatment.
Second system relies on radio frequency and includes a transmitter and antenna, which is carried outside the body and an implanted receiver.
Electrical current applied the dorsal surface of the spinal cord produces a state of analgesia.
Relieves pain in failed back syndrome, regional pain syndrome and peripheral neuropathy.
In patients with intractable spine/limb pain, spinal stimulation is associated with better pain reduction in medical therapy.
Can effectively manage neuropathic pain such as: brachial plexus neuropathy, complex regional pain syndrome, peripheral neuropathy, postherpetic neuralgia, post laminectomy syndrome, post-thoractomy syndrome, phantom limb pain, and radicular pain from cervical, thoracic, lumbar and sacral vertebrae.
Complications include lead migration, infection, epidural hematoma, paralysis, and rarely death.
A clinical trial is usually performed prior to the permanent placement of such a unit.
The temporary procedure utilizes a percutaneous lead connected to an external pulse generator and lasts for 3-7days, and if a 50% reduction in pain occurs the patient is considered for a permanent unit.
Proposed mechanisms of action: closes the gate by antidromic activation of large diameter afferent fibers, activation of the supraspinal loops relayed to the brainstem or thalamocortical systems providing ascending and discontinuing inhibition, activation of the pretectal nucleus which has descending pain inhibiting influence over lower segments, suppression of the wide-dynamic range neurons responsive to sensory input, and antidromic release of neuropathies in the periphery which may be related to modulation of small vessel diameter.
Can be used as implants to bilateral suboccipital region to control migraine headaches.
Can be interrupted by electromagnetic interference.
In the implanted SCS system the 2 to main components: the generator leads.
Energy from diathermy units can cause tissue damage at electrode site.
For most cases the placement of a spinal cord stimulating device is a two-stage process: stage one is a trial or temporary implant, and stage two is the implantation of the long term unit.
The trial procedure allows the patient and medical professionals to assess th likelihood that spinal cord stimulation will help the patient’s painful condition.
The trial involves placing the temporary spinal cord stimulator into the epidural space and is minimally invasive and an outpatient surgical procedure.
The temporary procedure does not usually require incision as the leads are place via epidural needles.
The trial lasts 3 to 10 days and the temporary is SCS is removed and a decision is made to replace it with the implant of long-term nature.
The implanted SCS system has two main components: the generator and the leads.
The lead or leads are placed in the dorsal epidural space usually via a minimally invasive surgical procedure under local anesthesia and intravenous sedation.
The variation exists in the type of lead depending on the patient’s anatomy, the pain diagnosis and the pain location.
From the spine, the leads are tunneled under the skin connect to a generator or battery.
The generator is surgically placed under the skin usually in the buttock, flank or abdomen.
Patients have some control over the process using a portable handheld remote control device on and off other and program functions.
These devices are generally rechargeable.
One of the most common indications for spinal cord stimulator is persistent radicular pain following lumbar spine surgery.
Among patients with chronic radicular pain after lumbar surgery the spinal cord burst stimulation affect compared with placebo stimulation, resulted in no significant difference in back pain related disability (Hara S),
