 Schwann cells are the principal glia of the peripheral nervous system (PNS).
Schwann cells are the principal glia of the peripheral nervous system (PNS).
Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle.
They are a type of glial cell that play a vital role in the peripheral nervous system (PNS).
Schwann cells are involved in many important aspects of peripheral nerve biology—the conduction of nervous impulses along axons, nerve development and regeneration, trophic support for neurons, production of the nerve extracellular matrix, modulation of neuromuscular synaptic activity, and presentation of antigens to T-lymphocytes.
They are responsible for myelinating and supporting the axons of peripheral neurons, forming the myelin sheath that insulates and speeds up nerve impulses.
In addition to their role as myelin-forming cells, Schwann cells also help to regenerate damaged nerves in the PNS.
When a nerve is damaged, Schwann cells can proliferate and form a pathway that enables the regrowth of the damaged axon.
This process is known as nerve regeneration, and it allows the PNS to recover from injuries and restore function.
Schwann cell dysfunction has been implicated in several neurological disorders, including peripheral neuropathies, Charcot-Marie-Tooth disease, and Guillain-Barre syndrome.
Schwann cells are highly plastic and have the ability to differentiate into other cell types in response to injury or disease.
Overall, Schwann cells play a crucial role in the proper functioning of the PNS and in the regeneration of damaged nerves.
The two types of Schwann cells are myelinating and nonmyelinating.
Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath.
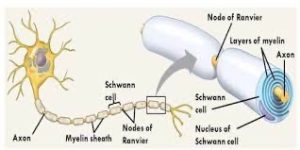
Structure of a typical neuron: At one end of an elongated structure is a branching mass.
At the center of this mass is the nucleus and the branches are dendrites.
A thick axon trails away from the mass, ending with further branching which are labeled as axon terminals.
Along the axon are a number of protuberances labeled as myelin sheaths.
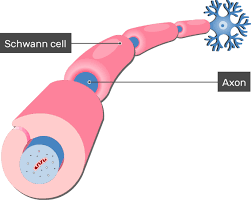
Schwann cells are a variety of glial cells that keep peripheral nerve fibers, both myelinated and unmyelinated alive.
In myelinated axons, Schwann cells form the myelin sheath.
The sheath is not continuous. Individual myelinating Schwann cells cover about 1 mm of an axon, equating to about 1000 Schwann cells along a 1-m length of the axon.
The gaps between adjacent Schwann cells are called nodes of Ranvier.
The nervous system relies on the myelin sheath for insulation and as a method of decreasing membrane capacitance in the axon.
The action potential jumps from node to node, in a process called saltatory conduction, which can increase conduction velocity up to 10 times, without an increase in axonal diameter.
Schwann cells are the PNS’s analogues of the central nervous system’s oligodendrocytes.
However, unlike oligodendrocytes, each myelinating Schwann cell provides insulation to only one axon.
Myelination greatly increases speed of conduction and saves energy.
Nonmyelinating Schwann cells are involved in maintenance of axons and are crucial for neuronal survival.
Myelinating Schwann cells begin to form the myelin sheath during fetal development and work by spiraling around the axon.
There may be as many as 100 myelin sheath revolutions.
The outermost layer of nucleated cytoplasm forms the neurilemma.
Schwann cells are known for their roles in supporting nerve regeneration.
If damage occurs to a nerve in the PNS, the Schwann cells aids in digestion of its axons by phagocytosis.
Schwann cells can guide regeneration by forming a type of tunnel that leads toward the target neurons.
Successful axons can reconnect with the muscles or organs they previously controlled with the help of Schwann cells.
Specificity is not maintained and errors are frequent, especially when long distances are involved.
Schwann cells have been connected to preferential motor reinnervation, as well.
If Schwann cells are prevented from associating with axons, the axons die.
Regenerating axons will not reach any target unless Schwann cells are there to support and guide them.
Schwann cells are essential for the maintenance of healthy axons, producing a variety of factors, including neurotrophins, and also transfer essential molecules across to axons.
SOX10 is a transcription factor active during embryonic development and evidence indicates that it is essential for the generation of glial lineages from trunk crest cells.
Neuregulin 1 (NRG1) acts to both promote the formation and ensure the survival of immature Schwann cells.
Myelin protein zero (P0) is a cell-adhesion molecule belonging to the immunoglobulin superfamily and is the major component of peripheral myelin.
Myelin protein zero (P0) constitutes over 50% of the total protein in the sheath.
Myelin protein zero (P0) has been shown to be essential for the formation of compact myelin.
Krox-20 is considered one of the master regulators of PNS myelination and is important in driving transcription of specific structural proteins in the myelin.
Charcot–Marie–Tooth disease (CMT), Guillain–Barré syndrome (GBS, acute inflammatory demyelinating polyradiculopathy type), schwannomatosis, and chronic inflammatory demyelinating polyneuropathy (CIDP), leprosy, and Zika Virus are all neuropathies involving Schwann cells.
