 Remote ischemic conditioning (RIC) is medical procedure that aims to reduce the severity of ischemic injury to an organ such as the heart or the brain.
Remote ischemic conditioning (RIC) is medical procedure that aims to reduce the severity of ischemic injury to an organ such as the heart or the brain.
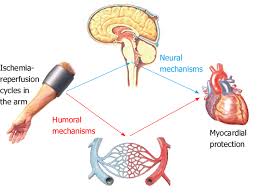
RIC uses transient cycles of limb ischemia, and reperfusion of the upper extremity to exert distant organ protection.
It is most commonly employed in the situation of a heart attack or a stroke, or during procedures such as heart surgery when the heart may temporary suffer ischemia.
It triggers the body’s natural protection against tissue injury.
It is still an experimental procedure.
RIC involves repeated, temporary cessation of blood flow to a limb to create ischemia in the tissue.
RIC activates the body’s natural protective physiology against reperfusion injury and the tissue damage caused by low oxygen levels.
Both humoral factors and upstream, nervous system activation, are implicated mechanisms of benefit
RIC essentially mimics the cardio-protective effects of exercise.
Exercise can be considered a form of RIC in which the stimulus is distant from the organ being protected.
More than 10,000 patients worldwide have completed clinical trials involving RIC, and another 20,000 are enrolled in ongoing trials.
In pediatric patients undergoing heart surgery patients treated with RIC prior to surgery exhibited less heart damage, as measured by the biomarker troponin, as well as less need for supportive drugs.
In multiple randomized controlled trials, remote ischemic conditioning reduced infarct size in ST-elevation myocardial infarction (STEMI) patients when used in the ambulance or emergency department.
When used as an adjunct therapy to primary percutaneous coronary intervention (PCI), or when used with thrombolytic drugs, RIC decreases infarct size.
In seven trials comprising 2,372 STEMI patients, infarct size was reduced by 17–30% on average, and the reduction was greatest, about 60%, in the largest infarcts.
A Danish study (CONDI-1), in which patients were treated in the ambulance, showed that those who received RIC did not show a decline in myocardial salvage index when they experienced a delay in treatment, while the control group experienced a significant decline in salvage index: extending the period in which medical treatment for heart attacks is most effective.
Long-term follow-up on STEMI patients treated with RIC found that the reduction in heart damage at the time of the heart attack resulted in clinical improvement four years later: major adverse cardiovascular and cerebrovascular event rates were reduced by 47% (13.5% vs. 25.6%).
Remote ischemic conditioning significantly reduced heart damage, as measured by troponin elevations, in four randomized controlled trials involving 816 elective PCI patients.
In a large study of RIC in elective PCI patients (CRISP study) treated with RIC prior to stenting showed a 62% reduction in troponin levels, less chest discomfort, and reduced six-month hospitalization rates.
Long-term follow-up of the CRISP study showed that this single RIC treatment resulted in a 35% reduction in six-year major cardiovascular and cerebrovascular event rates.
RIC has been shown to reduce contrast-induced nephropathy (CIN) and contrast-induced acute kidney injury.
5 randomized clinical trials comprising 480 patients. The first report showed a benefit in patients at extremely high risk of injury, those with Stage 3 or 4 kidney disease, diabetes, or heart failure:
reduced incidence of contrast induced nephropathy (CIN) with a 70% reduction, from 40% to 12%, with no patients in the treated arm needing in-hospital dialysis compared with 14% in the control group,
and a reduced six-week readmission rates (a 60% reduction, from 36% to 14%).
Similar protection was seen in cancer patients undergoing contrast-enhanced computed tomography (CECT): a 35% reduction in CIN across the population, and the patients at highest risk benefited the most, with a 60% reduction.
Clinical trials involving cardiac patients,show that RIC can protect the kidneys as well as the heart.
Systematic reviews and analysis of RIC in cardiac surgery, examining thirteen trials involving 891 patients, and found that RIC treatment reduced troponin levels by 21% to 49%.
72-hour cardiac troponin AUC showed a benefit from RIC treatment.
The effect of RIC on clinical outcomes in coronary artery bypass grafting (CABG), showed that RIC treatment reduced troponin levels and improved long-term morbidity and mortality: patients who received the anesthetic isoflurane benefited from the treatment, the anesthetic propofol blocked the effects of RIC.
Propofol abolishes the phosphorylation of STAT5, a key survival molecule that is activated by RIC.
A trial in high-risk CABG patients showed a reduced incidence of surgical acute kidney injury in RIC-treated patients (37.5% vs. 52.5%, a reduced need for dialysis, and shorter stays in the intensive care unit.
Because RIC modifies the expression of genes involved in inflammation, coagulation, and complement pathways, repeated treatments could aid recovery or prevent disease progression in a variety of chronic conditions.
Repeated daily RIC treatments lead to significant downregulation of neutrophil activation and proinflammatory responses and could reduce post-heart-attack inflammation.
RIC is thought to remotely recruit neuroprotective pathways, and its safety, feasibility, and low cost give it high potential in a wide variety of neurological conditions.
RIC not only confers protection against ischemia-reperfusion injury, but also increases cerebral blood flow, which may contribute to the neuroprotective effect.
In the RICAMIS Randomized Clinical Trial, a randomized trial that included 1893 patients with acute-moderate is chemic stroke, excellent neurologic function at 90 days in those randomize to RIC compare with usual care occur in 67.4% versus 62% difference that was statistically significant.
In a randomized trial of RIC in acute stroke patients, compared with standard treatment, RIC increased tissue survival after one month and reduced the risk of infarction in high-risk tissue.
Two randomized trials of RIC have also been conducted in patients with intracranial atherosclerotic stenosis (ICAS), a significant risk factor for stroke with a high risk of recurrence.
The first trial included patients under the age of 80 who had intracranial arterial stenosis of 50–99% and had experienced a stroke or transient ischemic attack (TIA) within the previous 30 days- evaluated the effects of 300 days of brief, repetitive, bilateral arm ischemic conditioning on stroke recurrence: the conditioning reduced the incidence of recurrent stroke from 23.3% to 5% at 90 days, and from 26.7% to 7.9% at 300 days; it also improved the rate of recovery and cerebral perfusion.
The second trial examined the effect of 180 days of RIC on symptomatic intracranial artery stenosis in people aged 80–95 years, as invasive stenting is not always suitable for elderly patients, and less-invasive methods are needed: RIC safely prevented stroke and TIA recurrence and reduced inflammation in these patients.
RIC initiated in the prehospital setting and continued in the hospital did not significantly improve functional outcome at 90 days in patients with acute stroke (Blaienfrldt RA).
Delayed cerebral infarction after subarachnoid hemorrhage is a major cause of morbidity: Two Phase I clinical trials have shown that RIC after subarachnoid hemorrhage is feasible, safe, and well tolerated, and can prevent delayed neurological deficits.
Traumatic brain injury (TBI) shares many pathophysiological pathways with acute stroke, and ischemic preconditioning increases the brain’s resistance to injury.
A randomized clinical trial in severe TBI also showed that patients who received RIC had lower levels of brain injury biomarkers.
The RIC stimulus can be applied to different tissues in the body, but either the arm or the leg leg may be used.
It is easier and more comfortable, most clinical trials use the upper limb.
RIC on one limb generates an equivalent response to RIC on two limbs, and that maximal benefit occurs at 4–6 cycles.
Pre-conditioning: RIC is applied within the hour prior to an intervention-elective cardiothoracic and surgical procedures.
Per-conditioning: RIC is applied at the time of the ischemic event-evolving heart attack, acute stroke, or trauma.
Chronic conditioning: RIC is applied daily for a period of time after an ischemic event-after a heart attack or stroke, or in chronic conditions such as peripheral vascular disease or ulcerative colitis.
Remote ischemic conditioning on the limb is mostly done by healthcare professionals, using a manual blood-pressure cuff and a stopwatch.
The standard RIC protocol, used in the majority of clinical trials, consists of four cycles of five minutes of inflation at 200mmHg, followed by five minutes of deflation.
The autoRIC Device, delivers four cycles of five minutes of inflation at 200mm Hg followed by five minutes of deflation to the upper limb.
The autoRIC Device is much easier to use.
