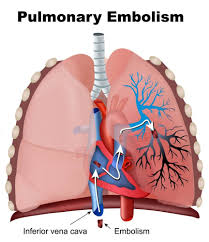
Pulmonary embolism is defined as an occlusion in the pulmonary arterial tree preventing blood flow distal to the occlusion.
PE is most frequently caused by thrombosis in a systemic blood vessel, usually in a deep vein of the lower extremity.
3% of surgical in-patient deaths, found in 24% of surgical patients at autopsy.
In the U.S. (1999) 140,00 fatalities and 400,000 non-fatal cases.
The annual incidence of PE worldwide is approximately one in 1000 persons.
Estimated 60-100: 000 deaths annually in the US.
Almost 20% of patients treated for PE dies within 90 days.
The three month mortality rate of patients with a pulmonary embolus has been reported to be 15%.
PE is not commonly the cause of death because it frequently coexists with other serious conditions such as cancer, sepsis, or illness leading to hospitalization, or with other events such as surgeries.
The true mortality associated with undiagnosed pulmonary embolism is estimated to be less than 5%, but recovery is associated with the complications, such as bleeding due to anticoagulants, recurrent venous thromboembolism, chronic thromboembolic pulmonary hypertension, and long-term psychological distress.
Estimated incidence 60-120 per 100,000 persons per year.
Approximately 25% of patients with incident pulmonary embolism experience sudden death.
A common cause of death after myocardial infarction and stroke.
Approximately 30% of patients die within the first year after diagnosis.
Approximately half of the patients who receive a diagnosis of PE jave functional and exercise limitations one year later: post pulmonary embolism syndrome.
The health related quality of life for patients with a history of PE is diminished as compared with matched controls.
In hospital mortality of 14% in a 90 day mortality rate of 20%.
Responsible for 5%-10% of all hospital deaths.
Clinical manifestations range from asymptomatic to hemodynamic collapse and death.
A metanalysis showed a history of dyspnea, immobilization come recent surgery, active cancer, hemoptysis, previous venous thromboembolism, or syncope are associated with an increased likelihood of PE.
PE can alter pulmonary gas exchange and cause hypoxemia, but hemodynamic compromise is the most significant contributor to worse prognosis.
The degree of pulmonary artery occlusion and associated vasoconstriction contribute to increased pulmonary vascular resistance, which increases right ventricular afterload and results in reduced left ventricular preload and decreased cardiac output.
The hemodynamic response to PE depends on the size of the occlusion in the presence of preexistent chronic right heart failure and left heart failure.
PE is a diagnostic challenge with an ultimate diagnosis made in fewer than 10% of patients being evaluated for PE.
Symptoms associated with PE are often non-specific and the process should be suspected in patients with chest pain or dyspnea without an obvious cause.
Approximately 5 to 10% of patients present to the emergency room, report chest pain and dyspnea as primary complaints.
Pulmonary embolism is diagnosed in only one patient for every 20 tested for the presence of PE when they present to an emergency department.
Non-invasive tests to rule out PE based on the assessed clinical probability of pulmonary embolism are extremely effective in safely reducing the use of CT, resulting in only 30 to 40% of the patients with suspected PE subsequently undergoing diagnostic imaging.
Patients may present with syncope.
Older age associated with higher annual incidence of PE; the incidence of PE is less than 50 per hundred thousand among younger people than 50 years compared with 350 per hundred thousand among older than 75 years.
The increased use of CT pulmonary angiography has resulted in increased incidence of PE, and it is now diagnosing smaller PEs or PE‘s that are not life-threatening and that could be left untreated without adverse outcomes.
PE consist of fibrin and red blood cells.
Approximately 70 to 80% of PEs begin as thrombi in the deep veins of the lower extremities or pelvis.
Approximately half of all deep veins in embolize to the lungs.
Patients with an unprovoked PE that occurs without the usual temporary conditions to predisposes to thrombosis, should be evaluated for the presence of blood factors that indicate a hypercoagulable state.
Approximately 6% begin in the deep veins of the upper extremities.
In the absence of shock, the mortality with anticoagulant therapy is in the range of 5%.
Thrombus formation is related to three factors: the Virchow triad-venous stasis, local hypercoagulability, and endothelial injury.
Other factors contributing to thrombi include local infection, extrinsic venous compression, intravenous catheters or devices, or trauma.
The incidence of fatal PE recurrence without treatment is as high as 5-35%.
95-98% of patients with acute pulmonary embolism have submassive obstruction of the pulmonary circulation and remain hemodynamically stable with a normal or above normal cardiac output.
Massive and submassive pulmonary emboli account for 5-10% and 20-25% of cases, respectively, and are associated with increased mortality.
A leading cause of maternal death with an overall incidence of 1.72 cases per 1000 deliveries, and accounts for approximately one death and every 100,000 deliveries.
These stable patients do well with anticoagulant therapy with the mortality rate of 6-8%.
Thrombolytic agents and pulmonary embolectomy are not indicated and inferior vena cava filters are not indicated unless there’s a major contraindication to anticoagulation.
Systemic thrombolysis improves pulmonary perfusion and reverses acute right heart failure in patients with pulmonary embolus, but is associated with an overall major bleeding risk of 10% and the 3-5% risk of hemorrhagic stroke.
Systemic thrombolysis is approved for massive pulmonary embolism, but its useless for massive pulmonary embolism remains controversial.
The mortality rate of massive pulmonary embolism is greater than 30% and has not significantly changed over decades.
Management of Acute Pulmonary Embolism Clinical Practice Guidelines
Factors influencing the assessment of risk after pulmonary embolism include: hemodynamic status, age, coexisting conditions, the extent and proximity of the clot, and the effects of the clot on right ventricular function, an estimate of the risk of bleeding with treatment options, especially intracranial hemorrhage.
Initiation of anticoagulation is recommended without delay in patients with high or intermediate clinical probability of PE while diagnostic workup is in progress.
If anticoagulation is initiated parenterally, low-molecular-weight heparin (LMWH) or fondaparinux is recommended over unfractionated heparin for most patients.
Rescue thrombolytic therapy is recommended for patients with hemodynamic deterioration on anticoagulation.
When thrombolysis is contraindicated or has failed, surgical pulmonary embolectomy is recommended; percutaneous catheter-directed treatment should be considered.
Extracorporeal membrane oxygenation (ECMO), along with surgical embolectomy or catheter-directed treatment, are considered in patients with refractory circulatory collapse or cardiac arrest.
When oral anticoagulation is started in a patient who is eligible for a non–vitamin K antagonist (VKA) oral anticoagulant an NOAC is recommended in preference to a VKA.
When patients are treated with a VKA, overlapping with parenteral anticoagulation is recommended until an international normalized ratio (INR) of 2.5 (range, 2.0-3.0) is reached.
NOACs are not recommended in patients with severe renal impairment, pregnant or lactating patients, or patients with antiphospholipid antibody syndrome.
Inferior vena cava (IVC) filters should be considered in patients with acute PE and absolute contraindications to anticoagulation, as well as in patients with recurrent PE despite therapeutic anticoagulation.
Routine use of IVC filters is not recommended.
No clinical evidence has demonstrated the placement of an inferior vena cava filter improves prognosis.
Systemic reviews of clinical trials report that placement of an inferior vena cava filter is associated with an absolute risk reduction of 5% of recurrent PE, an absolute risk increase of 2% of DVT, and had no effect on mortality.
Therapeutic oral anticoagulation for ≥3 months is recommended for all patients with PE.
Catheter-directed therapy is an alternative treatment for massive and submissive PE, utilizing intrapulmonary administration of low dose of thrombolytics and in some cases mechanical clot removal.
Catheter-directed therapy has reduced risk of major bleeding complications compared with systemic thrombolytic treatment.
Catheter directed therapy improves RV/LV ratio, measure of heart dysfunction, in padtients with massive and submissive pulmonary embolism more significantly than anticoagulation alone.
Occurs in 1-2% of cases of more than 23 million surgical procedures performed in the U.S. each year.
Incidence of approximately 3.5 per 1,000 hospital admissions, with a mortality of one per 1,000 admissions.
Estimated annual incidence in the U.S. is 1 episode per 1000 registered patients.
With the advent of pulmonary CT angiography the incidence of pulmonary embolus has increased, mortality has changed little, and case mortality has decreased.
The increased incidence of PE is occurring in a population of nonfatal emboli.
Prognosis depends on multiple factors: including hemodynamic stability, embolism size and location, and contraindications to anticoagulation or thrombolytic therapy.
Persistent hypotension or shock is the only widely excepted indication for systemic thrombolysis.
If left untreated pulmonary embolism associated with an overall mortality rate of up to 30%, compared with approximately 2 to 11% in those receiving anticoagulation.
Thrombi in the right heart are detected by echocardiograms in approximately 4% percent of patients with acute pulmonary embolism.
Right heart thrombi are most frequent in patients with massive pulmonary embolism, with the majority in the right atrium.
Thrombi are attached to the right atrium or right ventricle usually have a benign prognosis.
Free floating snake like thrombi are usually associated with massive unstable pulmonary embolism.
The risk of pulmonary embolus may actually be decreasing due to quality improvements in care such as increased prophylaxis against venous thromboembolism in hospitalized patients.
PE incidence has increased in surgical patients and has increased even more dramatically among obstetric patients, tripling in the years since the advent of CT pulmonary angiography (Wiener RS et al).
With the use of pulmonary angiogram CT scans there has been an epidemic of disease due to increased overdiagnosis of clinically unimportant cases.
Overdiagnosis PE by CT pulmonary angiogram explains the increased incidence of pulmonary embolus, decreased case fatality, and minimal changes immortality.
Trend for patients hospitalized with a pulmonary embolus indicates that in recent years there is a lower disease severity in patients admitted than in the past.
As many as 300,000 people die in the U.S. from acute pulmonary embolism each year.
Approximately 20% of patients die before the diagnosis or on the first day after diagnosis.
Of patients surviving a PE more than one day, up to 11% may die in the first 3 months, even with adequate therapy, although many die related to comorbidities.
Autopsy studies reveal that if examined meticulously more than half of patients have pulmonary emboli.
It is suggested that patients with subsegmental emboli detected by multi detector CT pulmonary angiogram are more likely to have complications from anticoagulation than adverse effects from the emboli (Donato AA et al).
Using clinical surveillance rather than anticoagulation therapy and patients will subsegmental pulmonary embolism and no deep vein thrombosis in the legs who have low risk of recurrent venous thromboembolism.
Obstructs the pulmonary artery with increased pulmonary artery pressure.
Ranges from an asymptomatic process that is incidentally discovered to massive embolism causing immediate death.
Half of the patients may present with chest pain, but the predominant symptom is dyspnea with associated tachycardia.
Presenting symptoms of PE are dyspnea, pain, andtacpnea, which are present in 85%, 40%, and 29% of patients with PE, respectively.
Thrombus releases vasoconstricting substances serotonin from platelets and histamine from tissue and generates thrombin in plasma all contributing to increases in pulmonary artery vasculature resistance and pulmonary artery pressure.
With increased pulmonary artery pressure the right ventricle afterload increases leading to tricuspid regurgitation, tricuspid valve dilation, increased right ventricle wall tension, and increased right ventricular oxygen demand.
With increased right ventricular pressure dilation and dysfunction occur with shifting of the interventricular septum producing underfilling of the left ventricle, decreased cardiac output, lowered blood pressure, compromised coronary perfusion and myocardial ischemic changes.
The extent of gas exchange and hemodynamic abnormalities caused by a PE, depends on the degree of pulmonary artery obstruction, the patient’s cardiopulmonary reserve and extent of neurohormonal adaptations to the insult.
Massive pulmonary embolism describes a subset of patients with acute pulmonary embolus, making up about 5% of patients, who present with hypotension, syncope, cardiogenic shock, cardiac arrest, respiratory failure and have a high mortality rate if aggressive care is not rapidly instituted.
Saddle PE refers to radiologic definition of thrombus that straddles the bifurcation of the pulmonary artery trunk often with extension into both the right and left main pulmonary arteries.
Saddle pulmonary embolism found in 2.6-5.4% of patients with PE.
Term massive PE is a hemodynamic definition and refers to a PE with shock and hemodynamics collapse.
With massive pulmonary embolism obstruction of 60 to 70% of the pulmonary circulation.
Shock is the prime indicator for advanced therapieswhich include: systemic thrombolysis, catheter directed thrombolysis. IVC filters, surgical embolectomy, or extracorporeal oxygenation.
Saddle pulmonary embolism can be present with a stable hemodynamic picture and benign clinical course.
Saddle pulmonary embolism associated with a mortality between 4.5-16%.
The mortality rates for patients with massive and submassive pulmonary embolism is about 16 % at 30 days.
Patients with saddle embolus tend to present with greater hemodynamic compromise, but they have a low in hospital mortality rate, similar to that of age, and severity matched non saddle pulmonary emboli cohorts.
Patients with saddle pulmonary emboli do you have a higher rate of DVT and higher risk of late decompensation.
Optical clinical management of patients with saddle pulmonary emboli is based on the initial and delayed hemodynamic status rather than on the location and extent of the clot.
Submassive PE accounts for approximately 20-25% of patients with acute
PE, and are identified as having normotension, and evidence of right ventricular dysfunction.
Patients with submassive PE have an increased risk of adverse outcomes and early mortality.
Patients with a pulmonary embolus with normal blood pressure and preserved right ventricular function are the majority of patients and have an excellent prognosis when treated with anticoagulation alone.
Increased levels of cardiac troponin correspond with greater risk of pulmonary embolus related death and all cause mortality.
Troponin elevations in pulmonary embolus provides evidence of myocardial injury, probably from right ventricular microinfarction.
Submassive pulmonary embolus can be diagnosed by detecting RV enlargement on enhanced CT of the chest.
RV enlargement on chest CT is defined by a RV diameter-LV diameter ratio in excess of 0.9.
RV enlargement on chest CT during an acute PE is associated with increased 30 day and three-month mortality.
Echocardiography is the best imaging study for detection of RV dysfunction, and allows for pulmonary artery pressure estimate measurement.
In pulmonary embolus patients with normal systolic pressure and echocardiograph evidence of right ventricular dysfunction have an increased risk of hypotension, shock, cardiac arrest and death.
Pulmonary embolism in patients that can be treated at home safely are low risk patients who are not in shock, do not require supplemental oxygen, parenteral narcotics for pain, and have other conditions requiring hospitalization.
The percentage of acute pulmonary embolism patients than are low risk is reportedly more than 50% of patients.
Home treatment of pulmonary embolism and includes low molecular weight heparin and oral anticoagulants.
Novel oral anticoagulants are approved for the treatment of pulmonary embolism without the need for parenteral heparin or low molecular weight heparin.
In patients with acute PE echocardiography should be performed in the presence of RV failure, elevated cardiac biomarkers, and unexpected clinical deterioration to identify patients that may benefit from initially more aggressive management.
Ninety day cumulative mortality approaches 21% among patients with PE and RV dysfunction, and 15% for patients with PE and preserved RV function.(Goldhaber SZ et al).
Death results from right ventricular failure.
The right ventricle has a major role in the pathophysiology of acute PE with direct physical obstruction of the pulmonary arteries, hypoxemia and pulmonary arterial vasoconstriction cause a rapid increase in pulmonary vascular resistance and right ventricular afterload.
Right ventricular afterload elevation may result in right ventricular hypokinesis and dilatation, with tricuspid regurgitation and ultimately RV failure.
Patients with an acute PE and RV failure may experience systemic hypotension, cardiogenic shock, and cardiac arrest.
Elevated RV diastolic pressures deviates the intraventricular septum for the left ventricle and impairs left ventricular filling.
Right ventricular pressure overload may also result in increased wall stress and ischemia by increasing myocardial oxygen demand.
Right ventricular pressure overload, ventilation-perfusion mismatch, increase in total dead-space , and right to left shunting results in dyspnea.
T-wave inversions in leads III and VI suggest acute pulmonary embolism.
Incomplete right bundle branch block with the rSR morphology supports the diagnosis of pulmonary embolism.
Anterior or precordial T-wave inversions are among the most common EKG signs of pulmonary embolism and indicates severe pulmonary embolism, as most patients have accompanying pulmonary arterial hypertension.
Mortality 30% in untreated patients and 2% in treated patients.
Majority of preventable deaths from pulmonary embolism ascribed to a missed diagnosis rather than to treatment failure.
The majority of patients can be safety treated at home, and do not have to be hospitalized.
Multiple studies have confirmed that low risk pulmonary embolism patients treated at home have a mortality rate, pulmonary embolism recurrence rate, and incidence of bleeding complications similar to pulmonary embolism patients who are hospitalized.
Patients treated for acute pulmonary embolism are at almost four times risk to die of recurrent thromboembolism in the next year as patients treated for deep vein thrombosis, 1.5% vs 0.4%, respectively.
30% of patients have a history of prior PE or deep vein thrombosis.
24% of patients with untreated deep vein thrombophlebitis develop pulmonary embolism, resulting in a mortality rate of 15%.
Lower limb DVT responsible in 90% of PE but only 10% clinically apparent.
If DVT not found in patients with pulmonary embolus it is likely the entire thrombus already had detached and embolized.
Thrombi in lower extremities and pelvic veins embolize through the IVC and right heart and obstruct thepulmonary arterial tree, with hemodynamic and gas exchange abnormalities.
Three-month mortality as high as 17%.
70-80% of fatal cases occur in nonsurgical patients.
Lower extremity ultrasound shows deep vein thrombosis in approximately 30% of patients.
Lung scan diagnostic in 25%-40% of patients.
Lung scan approximately a 10% false positive and false negative results.
In most patients in whom pulmonary embolism is suspected V/Q scans are either of low or intermediate probability, but the incidence of pulmonary embolism in these patients ranges from 10-40%: therefore V/Q scan limitation is its diagnostic uncertainty.
6-30% of patients have isolated thromboembolism of subsegmental pulmonary arteries.
And elevated alveolar-arterial oxygen gradient often present, but may be absent in 20-23% of patients.
Risk factors include: major surgery, surgical complications, lower extremity fractures, obesity, immobilization, age older than 40 years, previous thromboembolism, malignancies, hyperestrogenemic state, genetic or acquired hemostatic disorders and heart failure.
Responsible for as many as 40% of deaths after gynecologic operations.
Between 17% and 36% of upper extremity venous thromboses have been shown the cause pulmonary embolism.
Estimated that one in seven hospitalized patients who die, do so from pulmonary embolism.
A negative helical CT of the lung reliable excludes clinically important pulmonary embolism.
Computerized tomographic pulmonary angiography provides a clear result, either positive or negative, and can also detect other causes of respiratory symptoms.
After a negative CT thorax for pulmonary embolism additional imaging with pulmonary angiogram is not warranted.
As many as 26 to 32% of patients with pulmonary emboli have symptomatic deep vein thrombosis that can be confirmed by ultrasound and is an accepted surrogate for the diagnosis of pulmonary embolism in this population (Girard P).
Up to 50% of proximal deep venous thrombosis cases result in pulmonary embolism.
A potentially fatal complication of total joint arthroplasty.
In a study 13,133 patients who underwent total joint arthroplasty between 2000 and 2005 PE was diagnosed in 144 patients (1.1%)and shortness of breath (31.9%) and hypotension (30.6%) were the most frequent symptom and sign (Pulido L).
In the above study oxygen desaturation was the only indication for investigation of PE in 10% of patients, and pulse-oximetry reading of less than 90% was present in 63% of patients.
In the above study clinical signs and symptoms as well as severity of hypoxia did not correlate with size and location of PE.
Common clinical signs and symptoms, as well as changes in vital signs, have a low sensitivity for diagnosis of PE in patients undergoing joint arthroplasty (Pulido L).
A normal lung scan rules out pulmonary embolism.
A high probability scan established the diagnosis with a greater than 90% probability.
Up to 73% of episodes are clinically unsuspected or misdiagnosed.
Prevalence in suspected cases is only 25%-35%.
Typical abnormalities of arterial blood gases are hypoxia, hypocarbia and high alveolar-arterial oxygen gradient.
Arterial gases by itself are of limited value in the diagnosis of PE.
Diagnosis: the optimal diagnostic strategy for PE remains debated.
The conventional algorithm is the estimate the pretest probability, D dimer testing, chest imaging of CT pulmonary angiogram or pulmonary ventilation/perfusion scanning.
D-dimer is a fibrin degradation proteins fragment created when fibrin undergoes endogenous fibrinolysis.
Blood D-dimmer levels are increased in the presence of thrombosis.
When the threshold is 500 mg 500 ng/mL the test has a 97 to 100% negative predictive value for PE when is used as a diagnostic strategy to rule out PE without need for unnecessary chest imaging.
Standard diagnostic strategies for PE consist of three steps: evaluating clinical probability, D-dimer testing, and chest imaging if indicated.
PERC criteria: The pre-test probability of PE is to rule out criteria – 50 years or greater, pulse rate 100 per minute or greater, arterial oxygen saturation less than 95%, unilateral leg swelling, hemoptysis, recent trauma or surgery, prior PE or deep vein thrombosis, and exogenous estrogen use or an age adjusted D dimer cut off (age times 10 ng per/mLin patients 50 years or older) safely excludes PE.
The dual circulation from pulmonary and bronchial arteries make pulmonary infarct uncommon.
Obstruction is the most important cause of compromised physiology but the release of vasoactive and bronchoactive substances, such as serotonin, released from platelets can lead to ventilation-perfusion mismatches.
TREATMENT:
Goals of treatment with antithrombotic therapy is to minimize morbidity and mortality and prevent recurrence without causing excessive bleeding.
Patients should be stratified according to their risk for in hospital mortality as low, intermediate, or high risk when therapy is selected.
Approximately 5% of patients present with high risk PE, involving shock, end organ hypoperfusion, hypotension, arrhythmia, or cardiac arrest.
High risk patients should be evaluated for immediate reperfusion therapy by ruling out contraindications such as bleeding disorders, brain metastasis, and recent surgery.
Intravenous, systemic thrombolysis is the most readily available option for reperfusion, and include tenecteplase, or alteplase.
Alternative reperfusion approaches include surgical thrombectomy, and Katherine directed thrombolysis.
The simplified pulmonary embolism severity index classifies patients with pulmonary embolus at low risk for early mortality if all six items of the score or negative.
Intermediate risk is associated with echocardiographic or CT evidence of right heart strain, elevate cardiac biomarkers, such as troponins or brain natriuretic peptide, or both.
Systemic thrombolysis is not typically recommended for patients with intermediate risk, as the absolute reduction in the risk of hemodynamic decompensation of three percentage points is at the expense of a nine percentage point increase in the risk of major bleeding.
Patients with intermediate risk PE should receive anticoagulant therapy and be monitored for the one patient in 20 in whom shock may subsequently develop.
Low molecular weight heparin is the preferred immediate anticoagulant for patients with intermediate risk PE.
For patients with low risk PE without right ventricular strain and normal cardiac biomarkers can be treated with direct oral anticoagulants and assessed for outpatient treatment.
Direct oral anticoagulants are the first line treatment for most patients for the reducing the risk of recurrent venous thromboembolism, as they do not necessitate monitoring, and are effective as vitamin K antagonists in reducing the risk of recurrent venous thromboembolism:apixaban, edoxaban and rivaroxaban.
Vitamin K antagonists or preferred over direct oral anticoagulants in patients with advanced kidney or liver disease and in patients with the antiphospholipid syndrome, who are triple positive for lupus anticoagulant, anti-cardiolipin, and anti-beta2 glycoproteins I antibodies, have a very high antibody titers or have a history of arterial thrombosis.
Low molecular weight heparin should be used treat pregnant women with PE, since vitamin K antagonists and direct oral anticoagulants cross the placenta and are associated with adverse pregnancy outcomes.
The simplified pulmonary embolism severity score: age greater than 80 years, medical history of cancer, chronic cardiopulmonary disease, heart rate of greater than 109 bpm, systolic blood pressure less than 100 mmHg, and oxygen saturation by pulse oximetry on room air has less than 90%.
Patients with zero score have a 30 day mortality of one percent.
Patients who have at least one positive component in the score and a systolic blood pressure of at least 90 mm Hg are sat intermediate risk of a 30 day in hospital mortality risk approximately up to 10%.
Immediate anti coagulation primary therapy for vast majority of acute PE patients.
Other treatment options include: systemic fibrinolysis, catheter directed therapy, surgical embolic tommy, and IVC filter insertion.
Unfractionated and low molecular weight heparin therapy acceptable treatments, although not all regulatory agents have approved the latter for initiation of therapy.
The administration of DOAC’s is probably comparable with low weight molecular heparin for intermediate risk disease.
Oral Rivaroxaban is noninferior to standard therapy for initial and long term treatment of PE (EINSTEIN-PE Investigators).
Right ventricular dysfunction is associated with an increased risk of death, shock, or recurrent PE.
Patients with signs of right ventricular dysfunction on imaging and elevated cardiac biomarkers are defined as intermediate high risk patients.
Patients with hemodynamic instability, blood pressure lower than 90 mmHg that persists or is associated with in the organ hypoperfusion have an approximately 20% risk of a 30 day mortality, compared with 5% for non-high-risk PE.
In these high-risk patients, thrombolytic therapy is recommended.
Thrombolytic therapy is indicated for acute disease with cardiopulmonary compromise with systolic hypotension, carcinogenic shock or right ventricular dysfunction.
For patients with uncomplicated process thrombolytic therapy is not recommended.
No consistent association exists between the use of thrombolytic therapy and mortality, regardless of the patient’s cardiopulmonary status.
Use of thrombolytic therapy in pulmonary embolism associated with major hemorrhage.
Most devastating complication of thrombolytic therapy is intracranial hemorrhage.
Due to the risk of fatal bleeding, thrombolysis should not be prescribed for patients with active bleeding or for those at high risk for bleeding.
Intracranial hemorrhage reported in 2.1.% of 559 patients treated with thrombolytic therapy for pulmonary embolism (Dalen JE et al).
In a randomized controlled study comparing standard anticoagulation with primary thrombolytic therapy, there was no difference in mortality (Perlroth).
No evidence thrombolytic therapy decreases the mortality of acute PE in patients not in shock and having right ventricular dysfunction.
Thrombolytic therapy indicated in the presence of hemodynamic instability as massive pulmonary embolism associated with an 18% risk of death and the risk is higher when accompanied by cardiogenic shock at 30%.
Thrombolytic therapy indicated in PE with hypotension.
Right ventricular dysfunction in normotensive patients with PE may be an indication for thrombolytic therapy, although controlled trials do not show improvement in mortality and has an increased risk of hemorrhage compared to standard anticoagulation.
Chronic sequelae include post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension.
Patients with acute pulmonary embolism, should receive anticoagulant therapy for at least three months to reduce the risks of further embolization, thrombus extension, early recurrence of venous thromboembolism, and death.
Continuing anticoagulation indefinitely depends on whether the reduced risk of recurrent venous thromboembolism with continued anticoagulation therapy outweighs the risk of bleeding.
Among patients who have had a pulmonary embolism provoked by a major transient, reversible risk factor, such as surgery, confinement to bed, major, trauma or fracture, the long-term risk of venous thromboembolism recurrence is low and anticoagulation therapy can be stopped after three months.
If the pulmonary embolism was very large, or was associated with moderate dysfunction of the right ventricle, or if a patient has persistent residual symptoms, some recommend treatment extend to six months.
In patients with persistent provoking factors, such as cancer, antiphospholipid syndrome, or who have had previous episodes of unprovoked venous thromboembolism, the long-term risk of recurrence is high and indefinite anticoagulation is recommended.
Occult cancer is detected in 5.2% of patients within one year after diagnosis of unprovoked pulmonary embolism.
PADIS-PE study-Prolonged Anticoagulation During 18 Months versus Placebo After Initial Six Months Treatment for a First Episode of Idiopathic Pulmonary Embolism trial.
In the above study continued anticoagulation therapy in patients with a first unprovoked PE and with no major risk factors for thrombosis following an initial treatment of six months was randomly assigned to receive either 18 months of warfarin therapy or placebo- patients were assessed recurrent VTE or major bleeding.
The above stated showed that extending anticoagulation therapy for 18 months with warfarin significantly had fewer episodes of VTR 1.7% compared to13.5% for patients taking placebo.
In the placebo group there was 21 episodes of PE compared to 2 cases with warfarin.
In the above study the rate of bleeding was not statistically higher in patients taking warfarin.
