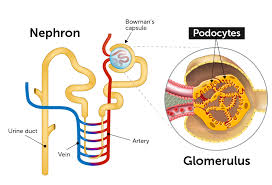
 Podocytes cover the outer surface of the glomerular basement membrane (GBM), closely enveloping the glomerular capillaries through extensions, meaning foot processes, that interdigitate with those of adjacent podocytes.
Podocytes cover the outer surface of the glomerular basement membrane (GBM), closely enveloping the glomerular capillaries through extensions, meaning foot processes, that interdigitate with those of adjacent podocytes.
Podocytes secrete and maintain the basement membrane of the glomerulus.
Podocytes are cells in the Bowman’s capsule of the kidneys that wrap around capillaries of the glomerulus.
Podocytes are present in lining the Bowman’s capsules in the nephrons of the kidney.
Podocytes possess a well-developed endoplasmic reticulum and a large Golgi apparatus.
These findings suggest a high capacity for protein synthesis and post-translational modifications.
There is a large number of multivesicular bodies and other lysosomal components seen in these cells, indicating a high endocytic activity.
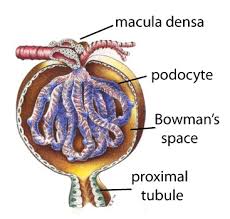
They make up the epithelial lining of Bowman’s capsule, the third layer through which filtration of blood takes place.
The Bowman’s capsule filters the blood, while retaining large molecules such as proteins.
Smaller molecules such as water, salts, and sugars are filtered at Bowman’s capsule, as the first step in the formation of urine.
Podocytes, are specialized epithelial cells that reside in the visceral layer of the capsule.
Blood flows through the capillaries of the glomerulus, where it is filtered by pressure.
Within each gomerulus, blood is filtered and moves under pressure through specialized capillaries which have 3 layers: fenestrated endothelial layer that retains red cells and molecules larger than approximately 6 to 8 nm, a basement membrane and a layer of podocytes.
Each podocyte has tentacle like foot processes tightly sealed to the basement membrane, tethered to it by adhesion molecules called integrins.
The proper functioning of this seal and that of the slit diaphragm, specialized junction between neighboring podocyte foot processes there is made up of structural proteins, such as nephrin, which extends from the surface of the podocyte into the slit diaphragm is critical to blood filtration process.
Pathogenic variants, in more than 60 genes encoding podocyte proteins, can cause kidney disease.
The filtering apparatus of the kidney is sensitive to circulating, inflammatory proteins, toxins, and antibodies.
Inflammatory insults caused by viral infection in integrin molecules of the foot process may become activated by the innate immune system, the soluble urokinase plasminogen activator receptor leading to changes in the nephron expression and the shortening of the slit diaphragm and eventually proteinuria.
Slit diaphragm changes induced by anti-nephron auto antibodies can compromise glomerular filtration.
Anti-nephron autoantibodies, wax and wain with disease activity, particularly in minimal change disease.
The podocytes are wrapped around the capillaries.
Blood is filtered through the slit diaphragm between the feet or processes of the podocytes.
The filtered blood passes out the proximal tubule.
The podocytes have long foot processes called pedicels, which wrap around the capillaries and leave slits between them.
For pedicels several proteins are required wrap around the capillaries and function.
The foot processes known as pedicels that extend from the podocytes wrap themselves around the capillaries of the glomerulus to form the filtration slits.
The pedicels, therefore, increase the surface area of the cells enabling efficient ultrafiltration.
When defects in these proteins, such as nephrin and CD2AP occur, their kidneys cannot function and predispose them to kidney failure later in life.
The slit diaphragm is formed by nephrin a zipper-like protein, with spaces between the teeth of the zipper, large enough to allow sugar and water through, but too small to let proteins through
CD2AP protein regulates the podocyte cytoskeleton and stabilizes the slit diaphragm.
Nephrin defects are responsible for congenital kidney failure.
Blood is filtered through these slits.
Blood flows through the capillaries of the glomerulus, where it is filtered by pressure.
The podocytes are wrapped around the capillaries.
Blood is filtered through the slit diaphragm between the feet or processes of the podocytes.
Blood flows in the afferent arteriole at and out the efferent arteriole.
Podocyte foot processes of neighboring cells are separated by filtration slits bridged by a membrane cell junction, called a slit diaphragm.
Foot processes are attached to the GBM by proteins that lead to cell matrix adhesion.
Podocytes allow for ultrafiltration of large volumes of fluid and small solutes necessary for clearance of toxic waste.
The interdigitating podocyte foot processes serve as buttresses against physical forces of hydrostatic pressure in the glomerular capillaries, compressing GBM‘s gel like structure.
The altered GBM acts as selective filter and restricts the permeability to macromolecules transported by diffusion and bulk flow.
The podocyte foot process architecture maximizes the area available for filtration of water and small solutes and provides mechanical resistance against blood pressure that compresses the GBM, preserving selectivity and preventing loss of albumin and other macromolecules.
Albumin in most of the plasma proteins components are retained in the bloodstream.
Mutations in genes expressed by podocytes as the genetic cause of albuminuria may occur in both familial and sporadic kidney disease.
NPHS1 gene encodes the immunoglobulin super family protein nephrin a major constituent of the slit diaphragm.
Nephrin molecules bridge the distance between adjacent podocyte foot processes to form a membrane like cell junction.
Nephrin Induces signaling to the podocyte cytoskeleton shaping its structure, mediating signal transduction.
Podocin a product of NPHS 2 interacts with nephrin at the slit diaphragm and organizes the lipid environment as a mechanicosensor at the filtration slit.
Podocin mutated protein in children and adolescents demonstrate steroid resistant nephrotic syndrome.
Podocyte express gene mutations can cause albuminuria, including cutoskeletal genes ACTN4 and INF2.
When podocytes are injured its cytoskeletal structure and intracellular junctions of the foot processes are altered, and that is called foot-process effacement.
Because podocytes are postmitotic cells with limited capacity for self renewal such podocyte damage results in irreversible damage and scarring of the renal filtration units.
Mutations in podocyte genes encoding mitochondrial proteins lead to mitochondrial dysfunction, impaired respiratory enzyme activity with secondary proteinuria.
Kidney diseases that lead to the nephrotic syndrome with excessive amounts of protein in the urine are usually caused by damage to podocytes specialized epithelial cells of the filtration barrier that regulates the filtration process.
Podocyte dysfunction is associated with genetic mutations, allergies, infections, lymphoid neoplasm, certain drugs, and auto immune diseases.
Many forms of podocyte injury are acquired, a many are antibody mediated.
Nephron is a key protein of the complex podocyte slit diaphragm architecture and has extensive signaling functions.
Circulating anti-nephrin auto antibodies are common with minimal change kidney disease or idiopathic syndrome and are markers of disease activity.
The podocyte area in contact with the glomerular basement membrane is called a podocyte foot process or pediclle.
Podocyte cells secrete and maintain the GBM.
Gaps between the foot processes are through which the filtrate flows into Bowman’s space of the capsule.
The space between adjacent podocyte foot processes is spanned by slit diaphragms consisting of a mat of proteins, including podocin and nephrin.
The foot processes have a negatively charged coat that repels negatively charged molecules such as serum albumin.
Some mesangial cells are in physical contact with capillaries, others are in physical contact with podocytes.
There is chemical cross talk among the mesangial cells, the capillaries, and the podocytes to fine-tune the glomerular filtration rate.
Podocyte injury can cause albuminuria, as indicated that numerous acquired diseases including: diabetic nephropathy, focal and segmental glomerulosclerosis, membranous nephropathy, hypertensive kidney disease, HIV associated nephropathy and lupus nephritis can all affect podocytes causing dysfunction of the filtration barrier and albuminuria.
Auto antibodies to podocyte antigens cause glomerular nephropathies.
Podocyte injury is associated with a more simplified architecture and the slit-diaphragm length is reduced, resulting in a reduction in the filtration slit area and a decline in glomerular filtration rate of water and small solutes.
Podocyte injury concurrently loses buttressing force and compressive force of the GBM is also reduced, which increases the permeability to albumin.
