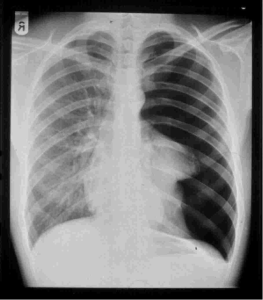
 A pneumothorax is an abnormal collection of air in the pleural space between the lung and the chest wall.
A pneumothorax is an abnormal collection of air in the pleural space between the lung and the chest wall.
Pneumothorax is associated with symptoms that include sudden onset of sharp, one-sided chest pain and shortness of breath.
In a minority of cases, there is a one-way valve that forms by an area of damaged tissue, and the amount of air in the space between chest wall and lungs increases; this is called a tension pneumothorax.
A tension pneumothorax can cause a steadily worsening oxygen shortage and low blood pressure, and can lead to a type of shock called obstructive shock.
Such a process can be fatal unless reversed.
Very rarely, both lungs may be affected by a pneumothorax.
A primary spontaneous pneumothorax occurs without an apparent cause and in the absence of significant lung disease.
A secondary spontaneous pneumothorax occurs in the presence of existing pulmonary disease.
Smoking increases the risk of primary spontaneous pneumothorax.
The main causes for secondary pneumothorax are COPD, asthma, and tuberculosis.
A traumatic pneumothorax can develop from physical trauma to the chest.
Diagnosis of a pneumothorax by physical examination alone is difficult: particularly in smaller pneumothoraces.
A chest X-ray, computed tomography (CT) scan of the chest, or ultrasound is usually used to confirm the presence of a pneumothorax.
Similar symptoms include a hemothorax, pulmonary embolism, and heart attack.
Management:
A small spontaneous pneumothorax will typically resolve without treatment and requires only monitoring.
With a larger pneumothorax, or if there is shortness of breath, the air may be removed with a syringe or a chest tube connected to a one-way valve system.
Occasionally, surgery may be required if tube drainage is unsuccessful, or repeated episodes occur, surgical treatments usually involve pleurodesis or pleurectomy (the surgical removal of pleural membranes).
About 17–23 cases of pneumothorax occur per 100,000 people per year.
Pneumothoraces are more common in men than women.
A primary spontaneous pneumothorax tends to occur in young adults, without underlying lung problems, and usually causes limited symptoms.
Chest pain and mild breathlessness are the usual predominant presenting symptoms.
PSPs more commonly occur during changes in atmospheric pressure,
It is rare for a PSP to cause a tension pneumothorax.
Secondary spontaneous pneumothoraces (SSPs), occurring in individuals with significant underlying lung disease tend to have more severe symptoms than in PSPs, as the unaffected lungs are generally unable to replace the loss of function in the affected lungs.
Secondary spontaneous pneumothoraces (SSPs) is usually associated with hypoxemia and may be observed as cyanosis.
Hypercapnia, which is the accumulation of carbon dioxide in the blood, may cause confusion and, if very severe, coma.
The sudden onset of dyspnea in someone with chronic obstructive pulmonary disease (COPD), cystic fibrosis, or other serious lung diseases should therefore prompt investigations to identify the possibility of a pneumothorax.
Traumatic pneumothorax commonly occurs when the chest wall is pierced, such as when a stab wound or gunshot wound that allows air to enter the pleural space, or because some other mechanical injury to the lung compromises the integrity of the involved structures.
Traumatic pneumothoraces have been found to occur in up to half of all cases of chest trauma, with only rib fractures being more common in this group.
The pneumothorax can be occult in half of these cases, but may enlarge.
A pneumothorax can be encountered in people already receiving mechanical ventilation for some other reason.
Upon physical examination, breath sounds may be diminished on the affected side, partly because air in the pleural space dampens the transmission of sound.
The conduction of vocal vibrations to the surface of the chest may be altered.
Percussion of the chest may be perceived as hyperresonant and vocal resonance and tactile fremitus can both be noticeably decreased.
The volume of the pneumothorax may not be well correlated with the intensity of the symptoms experienced by the victim, and physical signs may not be apparent if the pneumothorax is relatively small.
Tension pneumothorax is considered to be present when a pneumothorax (primary spontaneous, secondary spontaneous, or traumatic) leads to significant impairment of respiration and/or blood circulation.
Tension pneumothorax causes a type of circulatory shock, called obstructive shock.
Tension pneumothorax tends to occur in clinical situations such as ventilation, resuscitation, trauma, or in people with lung disease.
Tension pneumothorax is a medical emergency and may require immediate treatment.
Findings in people with tension pneumothorax are chest pain and respiratory distress, often with tachycardia and rapid breathing in the initial stages.
Other findings may include quieter breath sounds on one side of the chest, low oxygen levels and blood pressure, and displacement of the trachea away from the affected side.
Rarely, there may be cyanosis, altered level of consciousness, a hyperresonant percussion note on examination of the affected side with reduced expansion and decreased movement, pain in the epigastrium displacement of the apex heart beat and resonant sound when tapping the sternum.
Tension pneumothorax may also occur in someone who is receiving mechanical ventilation.
Spontaneous pneumothoraces are divided into two types: primary, which occurs in the absence of known lung disease, and secondary, which occurs in someone with underlying lung disease.
The cause of primary spontaneous pneumothorax is unknown.
Established risk factors include being of the male sex, smoking, and a family history of pneumothorax.
Smoking either cannabis or tobacco increases the risk.
Secondary spontaneous pneumothorax occurs in the setting of a variety of lung diseases: chronic obstructive pulmonary disease accounts for approximately 70% of cases.
The lung diseases may significantly increase the risk for pneumothorax.
Diseases of the airways COPD, especially when bullous emphysema is present, acute severe asthma, cystic fibrosis Infections of the lung Pneumocystis pneumonia (PCP) tuberculosis necrotizing pneumonia Interstitial lung disease Sarcoidosis, idiopathic pulmonary fibrosis, histiocytosis X, lymphangioleiomyomatosis Connective tissue diseases: Rheumatoid arthritis, ankylosing spondylitis, polymyositis and dermatomyositis, systemic sclerosis, Marfan’s syndrome and Ehlers–Danlos syndrome Cancer: Lung cancer, sarcomas involving the lung
In children, additional causes include measles, echinococcosis, inhalation of a foreign body, and congenital malformations: congenital pulmonary airway malformation and congenital lobar emphysema.
11.5% of people with a spontaneous pneumothorax have a family member who has previously experienced a pneumothorax.
Several hereditary conditions – Marfan syndrome, homocystinuria, Ehlers–Danlos syndromes, alpha 1-antitrypsin deficiency, and Birt–Hogg–Dubé syndrome have all been linked to familial pneumothorax.
A traumatic pneumothorax may result from either blunt trauma or penetrating injury to the chest wall.
The most common mechanism is the penetration of sharp bony points at a new rib fracture, which damages lung tissue.
Traumatic pneumothorax may also be seen in those exposed to blasts, even when there is no apparent injury to the chest.
Traumatic pneumothoraces may be classified as open or closed.
With an open pneumothorax, there is a passage from the external environment into the pleural space through the chest wall:sucking chest wound.
A closed pneumothorax is when the chest wall remains intact with a traumatic pneumothorax.
Pneumothorax was reported as an adverse event caused by misplaced nasogastric feeding tubes.
Medical procedures, such as inserting a central venous catheter into one of the chest veins or taking biopsy samples from lung tissue, may also lead to pneumothorax.
Positive pressure ventilation, either mechanical ventilation or non-invasive ventilation, can result in barotrauma leading to a pneumothorax.
Divers who breathe from an underwater apparatus are supplied with breathing gas at ambient pressure, which results in their lungs containing gas at higher than atmospheric pressure, and the breathing compressed air may develop a pneumothorax as a result of barotrauma from ascending just 3 ft while breath-holding with their lungs fully inflated.
Other features of decompression sickness are typically treated in a diving chamber with hyperbaric therapy; this can lead to a small pneumothorax rapidly enlarging and causing features of tension.
Pneumothorax is more common in neonates than in any other age group: incidence of symptomatic neonatal is estimated to be around 1-3 per 1000 live births.
Prematurity, low birth weight and asphyxia are the major risk factors, and a majority of newborn infant cases occur during the first 72 hours of life.
The lungs are fully inflated within the cavity because the pressure inside the airways is higher than the pressure inside the pleural space.
Despite the low pressure in the pleural space, air does not enter it because there are no natural connections to air-containing passages, and the pressure of gases in the bloodstream is too low for them to be forced into the pleural space.
A pneumothorax can only develop if air is allowed to enter, through damage to the chest wall or to the lung itself, or occasionally because microorganisms in the pleural space produce gas.
Once air enters the pleural cavity, the intrapleural pressure increases, resulting in the difference between the intrapulmonary pressure and the intrapleural pressure to equal zero, which cause the lungs to deflate in contrast to a normal transpulmonary pressure of ~4 mm Hg.
Chest-wall defects are usually evident in cases of injury to the chest wall, such as stab or bullet wounds.
In secondary spontaneous pneumothoraces, vulnerabilities in the lung tissue are caused by a variety of disease processes, particularly by rupturing of large air-containing lesions in cases of severe emphysema.
Areas of necrosis may precipitate episodes of pneumothorax.
Primary spontaneous pneumothorax (PSP) has been thought to be caused by small air-filled lesions (blend) just under the pleural surface, which were presumed to be more common in those classically at risk of pneumothorax.
Tall males are at higher risk of PSP due to mechanical factors.
Such blebs can be found in 77% of cases, compared to 6% in the general population without a history of PSP.
PSP may also be caused by areas of disruption in the pleural layer, which are prone to rupture.
Smoking may lead to inflammation and obstruction of small airways, which account for the markedly increased risk of PSPs in smokers.
Once air has stopped entering the pleural cavity, it is gradually reabsorbed.
Tension pneumothorax occurs when the opening that allows air to enter the pleural space functions as a one-way valve, allowing more air to enter with every breath but none to escape.
With a tension pneumothorax the body compensates by increasing the respiratory rate and tidal volume worsening the process: decreased oxygen levels and respiratory arrest eventually follow.
Tension pneumothorax is a medical emergency and may be treated before imaging.
In tension pneumothorax, X-rays are sometimes required if there is doubt about the anatomical location of the pneumothorax.
A plain chest radiograph, ideally with the X-ray beams being projected from the back
Posteroanterior, or PA films during maximal inspiration is the most appropriate first investigation.
The mediastinum contains the heart, great blood vessels, and large airways tend to be shifted away from the affected lung due to the pressure differences.
The size of the pneumothorax can be determined with a reasonable degree of accuracy by measuring the distance between the chest wall and the lung.
Smaller pneumothoraces may be managed differently.
An air rim of 2 cm means that the pneumothorax occupies about 50% of the hemithorax.
Measurement should be performed at the level of the hilum with 2 cm as the cutoff, or measurement should be done at the apex of the lung with 3 cm differentiating between a small and a large pneumothorax.
CT scanning can provide a more accurate determination of the size of the pneumothorax, but its routine use in this setting is not recommended.
Pneumothoraces are not uniform; some only form a pocket of air in a particular place in the chest.
Small amounts of fluid may be noted on the chest X-ray (hydropneumothorax); or may be blood (hemopneumothorax).
A CT scan is not necessary for the diagnosis of pneumothorax, but it can be useful In some lung diseases, especially emphysema: abnormal lung areas such as bullae have the same appearance as a pneumothorax on chest X-ray, and it may not be safe to apply any treatment before the distinction is made and before the exact location and size of the pneumothorax is determined.
In trauma, where it may not be possible to perform an upright film, chest radiography may miss up to a third of pneumothoraces, while CT remains very sensitive.
In presumed primary pneumothorax, CT may help to identify blebs or cystic lesions and in secondary pneumothorax, it can help to identify most of the causes.
Ultrasound is commonly used in the evaluation of people who have sustained physical trauma.
Ultrasound may be more sensitive than chest X-rays in the identification of pneumothorax after blunt trauma to the chest.
Ultrasound may also provide a rapid diagnosis in other emergency situations, and allow the quantification of the size of the pneumothorax.
The treatment of pneumothorax varies from discharge with early follow-up to immediate needle decompression or insertion of a chest tube.
Treatment is determined by the severity of symptoms and indicators of acute illness, the presence of underlying lung disease, the estimated size of the pneumothorax on X-ray, and – the personal preference of the person involved.
In traumatic pneumothorax, chest tubes are usually inserted.
If mechanical ventilation is required, the risk of tension pneumothorax is greatly increased and the insertion of a chest tube is mandatory.
Open chest wound should be covered with an airtight seal, as it carries a high risk of leading to tension pneumothorax: an Asherman seal is a specially designed device that adheres to the chest wall and, through a valve-like mechanism, allows air to escape but not to enter the chest.
Tension pneumothorax is usually treated with urgent needle decompression.
The needle or cannula is left in place until a chest tube can be inserted.
If tension pneumothorax leads to cardiac arrest, needle decompression or simple thoracostomy is performed as part of resuscitation as it may restore cardiac output.
Small spontaneous pneumothoraces do not always require treatment.
Small spontaneous pneumothoraces are unlikely to proceed to respiratory failure or tension pneumothorax, and generally resolve spontaneously.
Small spontaneous pneumothoraces: estimated size of the pneumothorax is small (defined as <50% of the volume of the hemithorax), there is no breathlessness, and there is no underlying lung disease.
Admission to hospital is often not required, as long as clear instructions are given to return to hospital if there are worsening symptoms.
Estimated rates of resorption are between 1.25% and 2.2% the volume of the cavity per day: complete pneumothorax would spontaneously resolve over a period of about 6 weeks.
Secondary pneumothoraces are only treated conservatively if the size is very small at 1 cm or less air rim, and there are limited symptoms.
Admission to the hospital is usually recommended.
Oxygen given at a high flow rate may accelerate resorption as much as fourfold.
In a large primary spontaneous pneumothorax (PSP) (>50%), or in a PSP associated with breathlessness, some guidelines recommend that reducing the size by aspiration is equally effective as the insertion of a chest tube:up to 2.5 liters of air, in adults, are removed.
This aspiration approach has been shown to be effective in over 50% of cases.
Aspiration may also be considered in secondary pneumothorax of moderate size (air rim 1–2 cm) without breathlessness.
All large pneumothoraces should be treated with a chest tube.
Moderately sized iatrogenic traumatic pneumothoraces may initially be treated with aspiration.
A chest tube, or intercostal drain, is the most definitive therapy for theb treatment of a pneumothorax.
They are typically inserted with local anesthetiv in an area under the axilla called, where damage to internal organs can be avoided.
In spontaneous pneumothorax, small-bore (smaller than 14 F, 4.7 mm diameter) tubes may be inserted.
Larger tubes do not have an advantage.
With traumatic pneumothorax, larger tubes (28 F, 9.3 mm) are used.
When chest tubes are placed due to either blunt or penetrating trauma, antibiotics decrease the risks of infectious complications when chest tubes are placed due to either blunt or penetrating trauma.
Chest tubes are required in PSPs that have not responded to needle aspiration, in large SSPs (>50%), and in cases of tension pneumothorax.
The chest tubes are connected to a one-way valve system that allows air to escape, but not to re-enter, the chest.
This may include a bottle with water that functions like a water seal, or a Heimlich valve.
Chest tubes are not normally connected to a negative pressure circuit, as this would result in rapid re-expansion of the lung and a risk of pulmonary edema.
The tube is left in place until no air is seen to escape from it for a period of time, and X-rays confirm re-expansion of the lung.
Negative pressure suction at low pressures of –10 to –20 cmH2O at a high flow rate may be attempted, particularly in PSP.
Failing this, surgery may be required, especially in SSP.
Bilateral pneumothorax is relatively common in people with pneumocystis pneumonia, and surgery is often required.
It is possible for a person with a chest tube to be managed in an ambulatory care setting by using a Heimlich valve.
Pleurodesis is a procedure that eliminates the pleural space and attaches the lung to the chest wall.
Good results are achieved with a thoracotomy with identification of any source of air leakage and stapling of blebs followed by pleurectomy of the outer pleural layer and pleural abrasion (scraping of the pleura) of the inner layer.
During the healing process, the lung adheres to the chest wall, effectively obliterating the pleural space: recurrence rates are approximately 1%.
Thoracoscopy, usually in the form of a procedure called video-assisted thoracoscopic surgery (VATS) is also effective, with a shorter in-hospital stays, less need for postoperative pain control, and a reduced risk of lung problems after surgery..
VATS may also be used to achieve chemical pleurodesis by insufflation of talc, which activates an inflammatory reaction that causes the lung to adhere to the chest wall.
Various agents may be instilled through the tube to achieve chemical pleurodesis, such as talc, tetracycline, minocycline or doxycycline.
Chemical pleurodesis tends to be worse than when using surgical approaches, but talc pleurodesis has been found to have few negative long-term consequences in younger people.
If pneumothorax occurs in a smoker, there may be benefits of smoking cessation to reduce risk of recurrence.
Those who have undergone pleurodesis may need two to three to recover.
Air travel is discouraged for up to seven days after complete resolution of a pneumothorax, and underwater diving is considered unsafe after an episode of pneumothorax unless a preventive procedure has been performed.
A preventative procedure such as thoracotomy or thoracoscopy with pleurodesis may be recommended after an episode of pneumothorax.
Not all episodes of pneumothorax require such interventions and such a decision depends largely on estimation of the risk of recurrence: These procedures are often recommended after the occurrence of a second pneumothorax.
The annual age-adjusted incidence rate of primary spontaneous pneumothorax (PSP) is thought to be three to six times as high in males as in females: 7.4 and 1.2 cases per 100,000 person-years in males and females, respectively.
Significantly above-average height is associated with increased risk of PSP – in people who are at least 76 inches (1.93 meters) tall, the rate is 200 cases per 100,000 person-years.
Slim build also seems to increase the risk of PSP.
The risk of a first spontaneous pneumothorax is elevated among male and female smokers by factors of approximately 22 and 9, respectively, compared to matched non-smokers of the same sex.
Individuals who smoke at higher intensity are at higher risk.
Men who smoke 10 cigarettes per day have an approximate 20-fold increased risk over comparable non-smokers, while smokers consuming 20 cigarettes per day show an estimated 100-fold increase in risk.
In secondary spontaneous pneumothorax, the estimated annual risk is 6.3 and 2.0 cases per 100,000 person-years for males and females,respectively, with the risk of recurrence depending on the presence and severity of any underlying lung disease.
Following a second episode has occurred, there is a high likelihood of subsequent further episodes.
The incidence in children is estimated to be between 5 and 10 cases per 100,000 person-years.
Death from pneumothorax is very uncommon, except in tension pneumothoraces.
British statistics show an annual mortality rate of 1.26 and 0.62 deaths per million person-years in men and women, respectively.
A significantly increased risk of death is seen in older patients and in those with secondary pneumothoraces.
