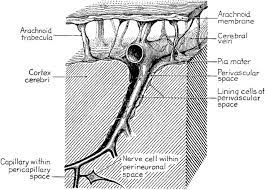
 A perivascular space, also known as a Virchow–Robin space (VRS) , is a fluid-filled space surrounding certain blood vessels in several organs, including the brain.
A perivascular space, also known as a Virchow–Robin space (VRS) , is a fluid-filled space surrounding certain blood vessels in several organs, including the brain.
It has a dispersive role for neural and blood-derived messengers, and has an immunological function.
One of the most basic roles of the perivascular space is the regulation of fluid movement in the central nervous system and its drainage.
They act as a sponge, and are essential for signal transmission and the maintenance of extracellular fluid.
The brain pia mater is reflected from the surface of the brain onto the surface of blood vessels in the subarachnoid space.
These perivascular cuffs in the brain are regions where leukocyte aggregations occur in patients with viral encephalitis.
Perivascular spaces vary in dimension according to the associated type of blood vessel.
In the brain most capillaries have an imperceptible perivascular space.
Some structures of the brain, such as the circumventricular organs,
have large perivascular spaces surrounding highly permeable capillaries.
The median eminence, a brain structure at the base of the hypothalamus, contains capillaries with wide perivascular spaces, as well.
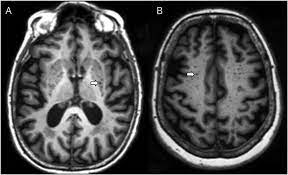
Perivascular spaces surround arteries and veins can usually be seen as areas of dilatation on MRI images.
An increase in these spaces may correlate with the incidence of several neurodegenerative diseases.
Perivascular spaces are gaps containing interstitial fluid.
The perivascular space spans between blood vessels and their host organ, such as the brain, which they penetrate and serve as extravascular channels through which solutes can pass.
Perivascular spaces are found in both the brain subarachnoid space and the subpial space.
Perivascular spaces surrounding arteries in the cerebral cortex and the basal ganglia are separated from the subpial space by one or two layers of leptomeninges, respectively, as well as the pia mater.
By virtue of the leptomeningeal cell layer, the perivascular spaces belonging to the subarachnoid space are continuous with those of the subpial space.
The direct communication between the perivascular spaces of the subarachnoid space and the subpial space is unique to the brain’s arteries, as no leptomeningeal layers surround the brain’s veins.
The spaces surrounding blood vessels in the subarachnoid space are not continuous with the subarachnoid space because of the presence of pia mater cells joined by desmosomes.
Perivascular spaces, especially around fenestrated capillaries, are found in the thymus, liver, kidneys, spleen, bones, and pineal gland.
Perivascular spaces are common within the brain circumventricular organs: subfornical organ, area postrema, and median eminence.
Large perivascular spaces are present around fenestrated capillaries, serve a dispersive role for brain- or bloodborne messengers.
Perivascular spaces may be enlarged to a diameter of five millimeters in healthy humans and do not imply disease.
Enlarged perivascular spaces can disrupt the function of the brain regions into which they project, and can occur on one or both sides of the brain.
Dilated perivascular spaces are categorized into three types/sites:
Type 1-in the lenticulostriate arteries projecting into the basal ganglia
Type 2 in the cortex following the medullary arteries
Type 3 in the midbrain.
Perivascular spaces are visualized by T2-weighted MRI images, and are
most commonly located in the basal ganglia and white matter of the cerebrum, and along the optic tract.
Many MRIs of other neurological disorders are similar to those of the dilated spaces:
cystic neoplasms
lacunar infarctions
cystic periventricular leukomalacia
cryptococcosis
multiple sclerosis
mucopolysaccharidoses
neurocysticercosis
arachnoid cysts
neuroepithelial cysts
Perivascular spaces appear as distinct round or oval entities with a signal intensity visually equivalent to that of cerebrospinal fluid in the subarachnoid space.
Perivascular spaces have no mass effect and is located along the blood vessel around which it forms.
Perivascular spaces are an integral part of the blood–brain barrier, separating the venous blood from the parenchyma of the brain.
Cell debris and foreign particles impermeable to the BBB will get through the endothelial cells, only to be phagocytosed in the perivascular spaces: T and B cells, and monocytes, giving this small fluid filled space an important immunological role.
Perivascular spaces play an important role in immunoregulation because they contain interstitial and cerebrospinal fluid, and have a constant flux of macrophages.
Perivascular spaces contain vasoactive neuropeptides (VNs), which regulate blood pressure and heart rate, have an integral role in controlling microglia.
Vasoactive neuropeptides serve to prevent inflammation by activating the enzyme adenylate cyclase which then produces cAMP., which aids in the modulation of auto-reactive T cells by regulatory T cells.
The perivascular space is susceptible space for when vasoactive neuropeptides are compromised and when their function is reduced in the space, immune response is adversely affected and the potential for degradation increases.
Inflammation by T causes astrocytes to begin to undergo apoptosis, due to their CD95 receptor, opening up the glia limitans and let T cells into the parenchyma of the brain.
This process is aided by the perivascular macrophages, these tend to accumulate during neuroinflammation and cause dilation of the spaces.
Perivascular spaces have a tendency to dilate.
Enlarged spaces have been observed most commonly in the basal ganglia, specifically on the lenticulostriate arteries, along the paramedial mesencephalothalamic artery and the substantia nigra in the mesencephalon, the brain region below the insula, the dentate nucleus in the cerebellum, and the corpus callosum, as well as the brain region directly above it, the cingulate gyrus.
Perivascular space dilation and lacunar strokes seen on MRI are the most commonly observed histological correlates of signaling abnormalities.
Perivascular space dilation is most commonly and closely associated with aging.
Perivascular space dilation is associated with symptoms and conditions that often affect the arterial walls, including vascular hypertension, arteriosclerosis, reduced cognitive capacity, dementia, and low post-mortem brain weight.
Dilation in young, healthy individuals can also be observed, with no observed association with reduced cognitive function or white matter abnormalities.
Extreme dilation of perivascular spaces in one hemisphere is associated with a non-specific fainting attack, hypertension, positional vertigo, headache, early recall disturbances, and hemifacial tics.
Symptoms associated with severe bilateral dilation of Virchow-Robin spaces include old ear pain, dementia, and seizures.
In most cases, there is in fact no mass effect associated with some perivascular space dilation.
When there is extreme dilation in the lower mesencephalon at the junction between the substantia nigra and cerebral peduncle, mild to moderate obstructive hydrocephalus can occur.
Associated symptoms with this process ranges from headaches, dizziness, memory impairment, poor concentration, dementia, visual changes, oculomotor abnormality, tremors, seizures, limb weakness, and ataxia.
Perivascular space dilation is a typical characteristic of several diseases and disorders: metabolic and genetic disorders such as mannosidosis, myotonic dystrophy, Lowe syndrome, and Coffin–Lowry syndrome; diseases or disorders of vascular pathologies, including cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, hereditary infantile hemiparesis, retinal arteriolar tortuosity and leukoencephalopathy, migraines, and vascular dementia.
Virchow-Robbins dilation is also associated with neuroectodermal syndromes: polycystic brains lesions associated with ectodermal dysplasia, frontonasal dysplasia, and Joubert syndrome.
A miscellaneous group of disorders typically associated with dilation that includes autism in children, megalencephalopathy, secondary Parkinson’s disease, recent-onset multiple sclerosis, and chronic alcoholism.
Virchow–Robin space ( RS) dilation is suspected to be related to:
mechanical trauma resulting from cerebrospinal fluid pulsation, elongation of ectactic penetrating blood vessels, and abnormal vascular permeability leading to increased fluid exudation, shrinkage or atrophy of surrounding brain tissue, perivascular demyelination, coiling of the arteries as they age, altered permeability of the arterial wall and obstruction of lymphatic drainage pathways.
The insufficient fluid draining and injury to ischemic perivascular tissue resulting in an ex vacuo effect have been suggested as possible causes for dilated VIrchow-Robbins spaces.
Analysis of VRS may distinguish dementia caused by arteriosclerotic microvascular disease from dementia caused by neurodegenerative disease.
S substantial amount of VRS in the substantia innominata, lentiform nucleus, and the caudate nucleus of the basal ganglia may implicate dementia due to arteriosclerotic microvascular disease, Ischemic Vascular Dementia.
These findings are, as opposed, to dementia due to neurodegenerative disease, specifically Alzheimer’s disease and frontotemporal dementia.
Thus, perhaps VRS dilation can be used to distinguish between diagnoses of vascular dementias and degenerative dementias.
VRS appear to be correlated with natural aging, MR imaging reveals a greater prevalence of VRS in those with Alzheimer’s.
Cerebral amyloid angiopathy (CAA), is a blood vessel failure often associated with Alzheimer’s disease that utilizes dilated VRS to spread inflammation to the parenchyma.
It has been hypothesized that the structure of VRS in the cerebral cortex may contribute to the development of Alzheimer’s disease.
VRS in the cerebral cortex may drain β-amyloid in interstitial fluid less effectively than VRS in the basal ganglia.
There is greater frequency of β-amyloid plaques in the cerebral cortex than in the basal ganglia of Alzheimer’s disease patients.
Abnormal dilation, along with irregular CSF pulsation are correlated with three or more risk factors for strokes.
There isa higher risk of stroke associated with dilated perivascular spaces in the elderly according to the Framingham Stroke Risk Score.
Other studies have concluded that the dilation of these spaces is a normal phenomenon in aging with no association with arterosclerosis.
Larger, and more prevalent perivascular spaces have been observed in those with MS,and other studies suggest that the inflammatory cells contribute to the demyelination that characterizes MS also attack the perivascular spaces.
Dilated perivascular spaces are common among the elderly and uncommon in children.
There is an association between both developmental delay and non-syndromic autism and enlarged or dilated perivascular spaces.
