A progressive central nervous system disorder caused by degeneration of dopaminergic neurons within the substantia nigra of the midbrain associated with degeneration in motor control.
The second most common neurodegenerative disorder and numerically the fastest growing neurologic disease, affecting 1% of people older than 60 years in high income countries.
Affecting 6 million people around the world.
Male: female ratio of approximately 2:1.
Its incidence rates range from 47 to 77 cases per hundred thousand persons 45 years of age or older and from 180 to 212 cases per hundred thousand persons 65 years of age or older.
Each day, an average of about 200 people are diagnosed with the condition in the United States,
Dopamine does not cross the blood brain barrier, but the immediate precursor levodopa is transferred cross the blood brain barrier.
Its pathological finding is the depigmentation of the substantia nigra and locus coeruleus with neuronal loss in the pars compacta of the substantia nigra.
Parkinson’s disease may be a disease of lysosomal abnormality.
Parkinsonism can also be produced by viral infections such as encephalitis or a number of toxins.
Many such toxins appear to work by producing reactive oxygen species.
Binding to neuromelanin by means of charge transfer complexes may concentrate radical-generating toxins in the substantia nigra.
Neuromelanin-containing neurons in the substantia nigra undergo neurodegeneration during Parkinson’s disease.
Motor symptoms of Parkinson’s disease are caused by cell death in the substantia nigra, which may be partly due to oxidative stress.
Motor symptoms of Parkinson’s disease are caused by cell death in the substantia nigra, which may be partly due to oxidative stress.
This oxidation may be relieved by neuromelanin.
Patients with Parkinson’s disease have 50% the amount of neuromelanin in the substantia nigra as compared to similar patients of their same age, but without Parkinson’s.
The death of neuromelanin-containing neurons in the substantia nigra, pars compacta, and locus coeruleus have been linked to Parkinson’s disease and also have been visualized with neuromelanin imaging.
MRI of the brain can identify changes in basal, ganglia or infra structures that are characteristic of other neurologic diseases involving Parkinsonism, but distinct from Parkinson’s disease, such as progressive supranuclear palsy, and multiple system atrophy.
Early symptoms occur prior to the movement disorder and include rapid eye movement sleep behavior disorder and decreased smell.
In time disease pathology progresses to the substantia nigra pars compacta and other midbrain and basal forebrain structures.
Advanced PD pathology progresses to the cerebral cortex with onset of cognitive impairment and hallucinations.
Cognitive changes such as visuospatial or executive dysfunction may be subjective symptoms and may precede motor symptoms.
Cognitive decline associated with mild cognitive impairment or Parkinson’s disease dementia develops in approximately 10% of patients annually.
Approximately 38% of patients with Parkinson’s disease and 89% of cases of dementia with Lewy bodies have Alzheimer associated pathological features.
PD protein aggregations are associated with dopamine producing cell death.
In parkinsonism there is decreased level of dopaminergic activity.
It is characterized by death of dopaminergic neurons in the substantia nigra.
Dopamine transporter single-photon emission computed tomography identifies the presynaptic dopamine neuronal dysfunction present in PD, and other neurodegenerative parkinsonisms are demonstrating reduced uptake of radioactive tracers that binds to dopamine transporters in the basal ganglia.
Dopamine transporter single-photon emission computed tomography is 98-100% sensitive and specific in detecting nigrostriatal cell loss in individuals with parkinsonism.
MRI is not usually helpful in the diagnosis of PD.
The pathological hallmark is the Lewy body.
A Lewy body is a neuronal inclusion consisting of alpha-synuclein protein aggregations.
The pathology of Parkinson’s disease is linked to Alpha-synuclein, small natively unfolded cytoplasm protein that can missfold and form aggregated polymers, which are a major component of Lewy bodies and Lewy neurites.
Rare genetic mutations of the SNCA gene encoding alpha-synclein cause autosomal dominant inherited Parkinson’s disease.
In the brain, Alpha-synuclein is highly expressed and its roles include vesicular transport and neurotransmitter release, including the release of dopamine.
It is posited in the progressive nature of Parkinson’s disease there is misfolded alpha-synuclein spreads from cells to cell and induces misfolding of native alpha-synuclein in a prion like fashion.
Some studies have shown an increased risk of Parkinson’s disease among patients with type two diabetes, as compared with people without diabetes, and there’s an association between alpha-synuclein aggregates, the pathological mark of Parkinson’s disease and insulin resistance in the brain.
Diabetes is a risk factor for Parkinson’s disease.
Some studies have shown had the prevalence of Parkinson’s disease was lower among patients with diabetes who were treated with GLP –1 receptor agonist or dipeptidyl peptidase-4 inhibitors, which increased GLP –1 levels, than among patients, who received other diabetes medications.
The treatment of diabetes with GLP-1 receptor agonists is associated with a reduction of more than 50% in the risk of new onset Parkinson’s disease.
GLP – 1 receptor activation reduces inflammation in the brain, a process that is central to the pathophysiology of Parkinson’s disease.
Some studies suggest an association between mild to moderate head injury and the onset of Parkinson’s disease.
Adrenergic blockers, statins, and non-steroidal anti-inflammatory drugs are associated with later age at onset in Parkinson’s disease.
Apoptosis and autophagy are involved in the process of depigmentation.
Is a result of dysfunction in motor and nonmotor thalamocortical loops in the parallel and segregated basal ganglia resulting in changes in rates of neuronal firing, neuronal firing patterns, and oscillatory brain cell activity.
Additional neurotransmitter systems are dysfunctional in PD and include serotonin, norepinephrine, and acetylcholine.
In parkinsonism, there is imbalance between levels of acetylcholine and dopamine in the brain, involving both increased levels of acetylcholine and degeneration of dopaminergic pathways (nigrostriatal pathway).
Manifests by motor and non motor symptoms.
Information suggests that neuroinflammation contributes to PD.
Microglia activation and increase in pro inflammatory factors are associated with the degeneration of dopaminergic neurons in PD.
Second most common neurodegenerative disorder behind Alzheimer’s disease.
Annual cost $2-20,000 per patient, about $6 billion/year.
Incidence increasing.
Estimated that approximately 930,000 people will be living with Parkinson’s disease in the US in 2020.
And uncommon process among individuals younger than 50 years and increases in prevalence with age, peaking between ages 85 and 89 years.
It is more common in men with a 1.4:1.0 male-to-female ratio.
Most cases are idiopathic, but known genetic and environmental contributions exist.
Prevalence increasing attributable to improved methods of detection, greater awareness of the disease, aging population, longer life expectancy, and possible increased environmental exposures to pesticides, solvents, metals, associated with industrialization.
Associated with exposure to trychloroethylene contaminant in ground water.
Pesticides, herbicides, and heavy-metal exposures are linked to an increased risk, whereas smoking and caffeine use are associated with decreased risks.
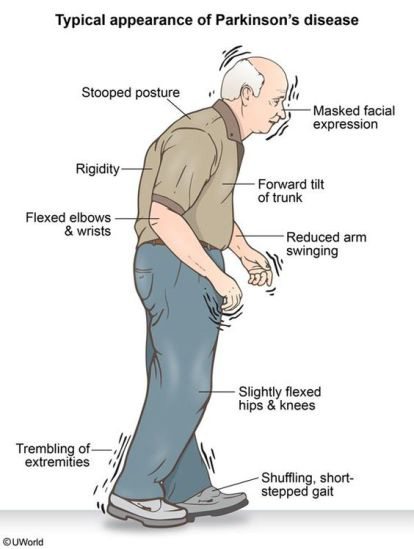
Manifests with any of the following: tremor at rest, bradykinesia, rigidity, postural instability, flexed posture and freezing.
Associated with a broad spectrum of motor symptoms including mask face, soft voice, tremor, rigidity, bradykinesia, dystonia, balance issues, shuffling, and small handwriting, and Nonmotor symptoms of depression, anxiety, impaired sleep, cognitive difficulties, and apathy, as well as problems with autonomic nervous system including constipation, gastrointestinal dysfunction, sexual dysfunction, and orthostatic hypotension.
Motor symptoms consist of movement and physical tasks: tremor, stiffness, slowness, and imbalance.
Non-movement symptoms can affect the G.I. and GU systems.
These prodromal non-movement symptoms include: rapid eye movement sleep behavior disorder, loss of smell, constipation, urinary dysfunction, orthstatic hypotension, excessive daytime sleepiness, and depression.
There are non-motor symptoms such as hyposmia, rapid eye movement sleep behavior disorder, depression, and constipation which maybe prodromal in Parkinson disease, but such symptoms increase as the disease process increases.
Sensory symptoms that include hyposmia occur in greater than 90% of patients, visual disturbances 22-78% of patients and somatosensory dysfunction and pain in 30-85%.
Rapid eye movement sleep behavior disorder is strongly associated with an increased risk of the subsequent diagnosis of PD.
Orthostatic hypotension presents in about 50% of patients and results in increased debilitation and the higher frequency of falling.
Urinary complaints include increased frequency and urgency.
Approximately 84% of patients with Parkinson�s disease have abnormal urinary function.
Nonmotor symptoms include: orthostatic hypotension, dysphasia, cognitive decline, psychiatric symptoms, pain, and constipation.
Depression and anxiety are present in approximately 35% of patients.
Apathy is seen with or without depression but is more commonly appreciated in patients who have cognitive decline.
Postural instability, freezing gate, and cognitive impairment are important non-levodopa responsive symptoms of PD and are significant risk factors for falls, which lead to hospitalization, disability in the evening.
Cholinergic system dysfunction in PD possibly contributes to postural instability and cognitive impairment.
About 1% -2% of individuals older than 60 years of age have Parkinson’ss disease.
Age is the most important risk factor.
Estimated that about 3% of the population over age 80 are effective.
Occurs in 0.3% of the population.
The crude prevalence rate of PD has been reported to range from 15 per 100,000 to 12,500 per 100,000, and the incidence of PD from 15 per 100,000 to 328 per 100,000, with the disease being less common in Asian countries.
Rarely seen in patients under age 40.
Parkinson’s disease occurs two years earlier on average in men than women.
Parkinson disease prevalence is increasing more rapidly than other neurological disorders.
Twice as many men as women develop the disease.
Estimated prevalence in US 1 million persons and 6 million worldwide.
Approximately 60,000 cases diagnosed annually.
Men more commonly affected then women, 1.6:1.
Peak onset between 55 and 65 years of age, with a mean age of onset 65 years.
Affects at least 100 persons in every hundred thousand.
Incidence in patients aged 55-65 0.3 per 1000 person-years.
Incidence in patients 85 years or older 4.4 .per 1000 person-years.
Prevalence in total population is 0.3% and for patients older than 60 years is 1%.
Parkinson’s disease is idiopathic and is the most common cause of Parkinsonism.
Living in rural areas and exposure to pesticides such as paraquat are risk factors.
Residential or occupational exposure to pesticides, has been associated with a dose dependent, 40% or greater risk of Parkinson’s disease.
Patients with the highest exposure to nitrogen dioxide, were 40% more likely to have Parkinson’s disease.
Nitrogen dioxide is commonly released by vehicles and power plants.
Smoking and coffee drinking appear to be protective.
Cardinal features: resting tremor, bradykinesia, rigidity, and gait disturbance.
Most cases result from a genetic susceptibility and environmental exposures.
One of every three patients with PD will become unemployed within one year, and most will be unemployed by five years.
Annual risk of hospitalization exceeds 30%.
On average patients with PD spend $1000-6000 per year on medications.
Etiology: Idiopathic:hereditary-90:10%.
Genetic mutations identified, especially among individuals younger than 50 years.
5-10% of idiopathic cases are familial.
Typical presentation with unilateral or assymetrical motor signs.
Genes associated include alpha-synuclein, LRRK2, parkin, DJ-, and PINK1.
Genetic variants with large effect sizes is identified in approximately 20% of patients.
Autosomal dominant Parkinson’s disease is seen in approximately 1 to 2% of cases and up to 40% of familial cases.
GBA1 encoding glucocerebrosidase which is presently 5 to 15% of cases most commonly in Ashkenazi Jews of North African ancestry.
In individuals without a strong genetic risk factor, heritability is estimated to be 20 to 30% suggesting a contribution to non-genetic factors.
Pathology reveals neuronal loss with depigmentation of the substantia nigra and the presence of Lewy bodies.
2062
Neuronal loss is seen in the basal nucleus and the dorsal motor nucleus of the vagus nerve.
Lewy bodies are intracytoplasmic inclusions containing alpha-synuclein.
Several alpha-synuclein (SNCA) mutations cause the disease by gene overexpression causing amino acid substitutions and configurational changes in protein.
While SNCA gene mutations account for less than 1% of cases of Parkinson’s disease abnormal aggregation of the SNCA protein is present in all such patients.
Common SNCA variants may be associated with Parkinson’s in the general population not associated with the rare mutations.
SNCA protein is the principal component of Lewy bodies.
Lewy bodies restricted to the subcortical structures as the substantia nigra in patients with Parkinson’s disease without dementia.
At autopsy Lewy bodies, intraneuronal accumulation of misfoled alpha – synuclein nuclear protein, and Lewy neurites collectively termed Lewy pathology is present up to 90% of clinically defined cases of Parkinson’s disease:selectively affecting brainstem nuclei and substantial nigra, peripheral autonomic regions, myenteric plexus, sympathetic ganglia, skin, autonomic nervous system, limbic, and neocortical regions.
Early in the disease process dopamine deficiency is the predominant neurochemical abnormality.
Vitamin B 12 is lower in patients with PD and low levels are associated with peripheral neuropathy, cognitive impairment and more rapid progression of disease.
As disease progresses involvement of nondopaminergic brain regions results in levodopa resistant motor and nonmotor symptoms.
Overtime patients with Parkinson’ss disease require more frequent levodopa doses in addition to higher doses, as they lose their long-duration response to dopaminergic medication, and their short duration response decreases due to disease related changes in the brain.
Involvement of motor system causes tremor, rigidity, postural instability and slow movements.
Eventually, patients have difficulty rising from a chair.
Patients have a decrease in arm swing, usually asymmetrically and a length of a patient’s stride diminishes and the arm swing may disappear altogether.
Patients lose the ability to turn on the pivot but turn en block, using multiple small steps to turn.
Patients may develop propulsion or retropulsion.
The patient’s trunk may get a head of his feet and he may require to take small running steps to regain balance, termed festination.
Falling occurs late in the disease.
Myerson’s Sign is a medical condition where a patient is unable to resist blinking when tapped on the glabella, is common in PD.
A reduced rate of blinking is associated with Parkinson’s disease.
Clinical subgroups
Tremor-dominant 8%
Akinetic-rigid. 26%
Mixed. 66%
Symptoms become apparent when approximately 60% reduction in dopamine in the substantia nigra pars compacta and about 80% reduction of dopamine in the striatum.
Natural history ranges from slow to rapid progression.
Two major subtypes: 1-dominated by tremors and 2-manifested by gait and postural difficulties.
Tremor subtype associated with relatively normal mental status, slower onset of progressive disease and younger age at onset.
Initially, tremor is typically seen in one extremity, and sometimes involving only one finger or the thumb.
Parkinson’s tremor is slower at 4-6 Hz than in classic essential tremor at 8 to 10 Hz, and is most prominent when the limb is in the posture of repose.
Complete relaxation frequently abolishes the tremor.
The tremor is suppressed with movement.
Less commonly the head, jaw, and tongue may be involved.
For some patients classic tremor is the only manifestation of the disease.
Older individuals with mainly akinesia, rigidity and postural instability have a more aggressive clinical course than younger patients.
There are three subtypes of PD:
PD subtypes include mild motor predominant, intermediate and diffuse malignant.
Mild motor predominate occurs in younger patients, mild motor and non-motor symptoms, slow progression, good medication response.
Intermediate subtype: Age intermediate in onset, and symptomatology, and moderate to good response to medications.
Diffuse malignant: motor symptoms accompanied by rapid eye movement sleep behavior disorder, mild cognitive impairment, orthostatic hypotension, worse levodopa response, more prominent dopaminergic dysfunction on dopamine transporter single-photon emission CT, more atrophy changes in the MRI, low beta amyloid, and Beta/tau ratio in the CSF, and rapid progression.
Patients with malignant Parkinson disease have earlier and more severe symptoms, poor response to treatment medications, and rapid progression.
Mild motor predominant form makes up 49-53% of cases, followed by the intermediate form of 35-39%, and the diffuse malignant form is least common with 9-16%.
The diffuse malignant group have a mean time from diagnosis to 1st mile stone of falling, wheelchair dependence, dementia, or nursing home placement of 3.5 years, compared with 8.2 years for the intermediate form and 14.3 years for the mile motor-predominate form.
Main survival after diagnosis is 8.1 years for the diffuse malignant group, 13.2 years for the intermediate subtype, and 20.2 years for the mild motor predominant form.
Anosmia, the loss of the sense of smell, occurs in as many as 90% of patients with Parkinson’s disease and may precede symptoms by many years.
Loss or reduction of smell (anosmia) is common in Parkinson’s, with up to 95% of people experiencing it to some degree.
It can be one of the earliest symptoms, and people often report experiencing loss of smell before they even have any difficulties with movement.
Moderate to strong protective effect of aspirin and nonaspirin.
Risk increased in those exposed to herbicides, pesticides, rural living and drinking well water.
Risk increased with history of head trauma, and constipation.
The use of pesticides rotenone and paraquat is associated with increased risk, with a 2.5 times higher odds of developing the disease then control patients.
Risk reduced in smokers and caffeine consumption
Postural instability and impaired gait subtype associated with more dementia, bradykinesia and more aggressive clinical course.
Bradykinesia accounts for most of the signs and symptoms of the disease.
Bradykinesia refers to the slowing of movement and simplification of complex motor tasks.
Spontaneous movement is decreased in Parkinson’s disease, manifesting as the masked faces.
The blink rate is decreased, the eyes are more open, giving the appearance of staring.
Bradykinesia appears with progression of disease, and initially muscle slowing may involve muscles of 1 extremity, but eventually the process becomes bilateral and involves the body and trunk.
Bradykinesia results in a slow shuffling gait, with stooped posture.
Resting tremor, soft voice, small handwriting, shuffling, rigidity, bradykinesia and impaired balance are motor manifestations.
Up to 50% of patients will have symptoms refractory to medications and will experience drug-induced dyskinesias.
40% of patients with Parkinson’s disease experience dyskinesias after4-6 years of levodopa treatment, typically at times of high levodopa concentrations.
Dyskinesia is associated with a longer duration of Parkinson disease and higher levodopa doses.
Severe dyskinesia is reported in about 3% of patients with Parkinson’s disease treated with levodopa.
Patients, in whom levodopa was initiated early are more likely different to develop dyskinesias.
Levodopa-carbidopa enteral suspension treats motor fluctuations and dyskinesias and is administered continuously via a pump through the percutaneous endoscopic transgastric jejunostomy: resulting in a more continuous plasma levodopa level then oral dosing.
For Parkinson disease dementia rivastigmine is clinically useful with slight improvement in function.
Donepezil and galantamine are possibly useful, but no evidence supportsmemantine for cognitive impairment.
Selective serotonin reuptake inhibitors, selective serotonin norepinephrine reuptake inhibitors, tricyclic antidepressants and pramipexole, a dopamine agonist are useful for depression in some patients with Parkinson’s disease.
Non-pharmacological management such as cognitive behavioral therapy and trans cranial magnetic stimulation may be useful for treating depression and Parkinson’s disease.
Overactivity of the globus pallidus internus and the subthalamic nucleus is believed to be part of the pathophysiology.
Risk of death from any cause is nearly doubled with diagnosis, regardless of duration of the disease.
A meta-analysis found that people typically live 6.9-14.3 years after diagnosis of Parkinson�s disease.
Parkinson disease-related deaths increase with age and because of death in patients are similar to causes in non-Parkinson’s disease cohorts.
Parkinson disease associated deaths occur before advanced disease stage.
When individuals die of Parkinson’s disease related symptoms, aspiration pneumonia is the most common cause.
A number of diseases states can cause Parkinson-like syndrome and include: post-infectious encephalitis, antipsychotic drugs, antiemetic drugs, lithium, carbon monoxide, methanol, ethanol, cerebrovascular insufficiency, head trauma, hypothyroidism, brain tumor, normal-pressure hydrocephalus, Wilson’s disease, Huntington’s disease, Lewy body disease, progressive supranuclear palsy, Shy-Drager syndrome, Alzheimer’s disease and neurological deterioration.
Parkinson’s-like syndromes are usually associated with atypical findings, paucity of tremor and poor response to levodopa therapy.
Exercise and physical activity delays the deterioration of motor functions and prolongs functional independence in Parkinson’s disease.
Studies of large populations have shown that people who exercise are less likely to develop Parkinson’s.
Bradykinesia manifests as difficulties with fine motor movements of the fingers, decreased arm swinging, hypophonia and impaired facial expression (hypomimia).
Facial muscles move less, and the face is less emotive.
Hand movements become more restricted, and alternating movement becomes difficult with frequent freezing.
Freezing refers to intermittent arrest of motor function.
Writing becomes small and cramped.
The mouth often stays slightly open and speech become softer and monotone with words running together.
Swallowing ability is reduced with mechanics of swallowing being affected, resulting in sialorrhea.
Sialorrhea is not due to increased salivary production but due to the inability to efficiently handle saliva.
Cogwheel rigidity, a rachet like resistance to passive movement commonly occurs.
Associated with cognitive slowing and impaired memory.
About 20% of patients do not have resting tremor.
Symptoms such as abnormal rapid eye movement sleep behavior, olfactory loss, anxiety, and autonomic dysfunction, such as constipation, may develop 20 or more years before recognizable PD (Savica R et al).
Parkinson’s disease patients are known to experience severe constipation due to GI tract dysfunction years before the onset of motor movement complications, which characterizes Parkinson’s disease.
Dysautonomia is apparent in virtually all patients and includes constipation, which may be a very early symptom.
Gastrointestinal complaints including bloating, nausea and abdominal discomfort.
Frequently patients experience autonomic symptoms of urinary urgency, constipation, impaired thermal regulation, and orthostatic hypotension.
Autonomic symptoms that include constipation, orthostatic hypotension, and urinary dysfunction increase in frequency as the disease progresses.
Automatic symptoms including anxiety occurs in 60% of patients, apathy in 60%, and depression 35%.
Nonmotor manifestations: depression, anxiety, sleep abnormalities and autonomic dysfunction may occur early or late in the disease.
Associated with frontal lobe dysfunction, memory difficulties, sleep disorders, sleep apnea, sexual dysfunction, orthostasis, and digestive problems.
Advanced disease may be associated with frozen gait, postural imbalance, falls and swallowing problems and impaired speech.
Advanced disease is characterized by severe off periods, dyskinesias, impaired cognition, hallucinations, apathy, sleepiness, autonomic dysfunction, dysphasia, dysarthria, posture and balance impairments, freezing of gait, recurrent falls, and disability requiring help for activities of daily living.
In advanced Parkinson’s disease therapy has little or no benefit because the changes caused by the dysfunction are outside the dopaminergic pathways.
Associated with depression in about 50% of cases.
One third of patients lose employment within 1 year of diagnosis.
Meta-analyses reports a reduced aggregate risk of cancer in patients with PD.
Dementia can develop, and has a prevalence of 30-40%.
The highest rates of dementia are found in men 60-80 years of age.
Affects approximately 1% of all Americans older than 60 years.
Men:women, 3:2.
Average age of onset 55 years.
Onset usually insidious.
About 70% present with resting tremor of the upper extremities.
Tremor is one of the first symptoms to appear, but may be absent or mild.
Some patients have functionally disabling action tremor.
The Braak staging system outlines the temporal course of PD (Braak H et al).
No currently available test that is both sensitive and specific for the diagnosis.
PD diagnostic criteria include: rest tremor, improvement with dopaminergic therapy, the presence of levodopa induced dyskinesias, or the presence of either olfactory loss or cardiac sympathetic denervation on myocardial scanning.
Air pollution from the burning of fossil fuels may increase the risk of Parkinson’s disease.
Patients with the highest exposure to nitrogen dioxide, were 40% more likely to have Parkinson’s disease.
Nitrogen dioxide is commonly released by vehicles and power plants.
Evidence suggests exposure to pollutants and chemicals in the environment plays an important role in the development of Parkinson’s disease.
Air pollution is responsible for shortening people’s lives worldwide on a large scale, and the world is facing an air pollution pandemic.
Exposure to metals, type two diabetes, certain inflammatory disorders, and infections have been suggested to increase the risk of Parkinson’s disease.
Decreased risk for Parkinson’s disease is noted with cigarette smoking, caffeine consumption and increased physical activity.
Parkinson’s disease has become much more common , now affecting 6 million people around the world.
Each day, an average of about 200 people are diagnosed with the condition in the United States,
The disease is most prevalent in regions with high levels of air pollution, such as Europe and North America.
Rates are lowest in non-industrialized areas of Africa.
Parkinson’s diseases rates are growing fastest in parts of the world that are being developed quickly, such as China and India.
Previous studies have also linked Parkinson’s with exposure to pesticides and industrial chemicals.
People who had been exposed to the highest levels of nitrogen dioxide over a 5-year period were about 40% more likely to have Parkinsons disease than were people with the lowest levels of exposure.
The study found no link between the disease and five other pollutants, including ozone, particulate matter, and sulfur dioxide.
The environment, and nitrogen dioxide specifically, contribute to Parkinson’s disease.
A 2016 study in Denmark linked exposure to traffic-related pollution with a higher risk of Parkinson’s.
People often lose their sense of smell 10 to 20 years before they have the tremors and other classic symptoms of PD.
Suggesting breathing in pollutants and chemicals may damage the olfactory system before the brain begins to decline.
All current treatments are symptomatic.
When symptoms affect the quality of life, treatment is initiated.
There is no compelling evidence that starting treatment early has any impact in the progression of disease.
Parkinson disease treatment of motor symptoms are primarily dopamine-based.
Levodopa, dopamine agonist, and monoamine oxidase-B inhibitors are useful initial treatments.
MAO-B Inhibitors and dopamine agonists are associated with less robust symptom relief than levodopa but have a lower dyskinesia risk.
MAO-B inhibitors and dopamine agonists are used as adjuncts to levodopa and dopa
are dosed 1-3 times per day throughout the disease course and requires more frequent dosing overtime.
MAO-B inhibitors block enzymes that degrade dopamine, prolonging the benefits of levodopa.
Patient initiated levodopa treatment early in diagnosis had persistent mobility benefits and performance of activities of daily living.
No treatment confers neuroprotection.
Management consists of drug therapy, surgery, physical and speech therapy.
Physical therapy, physiotherapy, occupational therapy, and speech therapy are useful in the treatment of PD.
No drugs slow the progression of the disease.
Treatment is individualized to specific symptons, signs, dysfunctions, age, stage, level of activity and productivity.
Management is guided by impairment of dominant hand function, degree of functional impairment, and disease effect on activities of daily living, social relationships, and degree of bradykinesia and gait disturbance.
Dopamine replenishment is the fundamental basis for medical treatment, as motor and nonmotor symptoms, reflecting the loss of nigrostriatal dopaminergic system, often respond to this management.
While levodopa is the mainstay of pharmacological therapy other dopaminergic medications can be used as monotherapy or in conjunction with levodopa and include: dopamine agonists, amantadine, anticholinergics, monoamine oxidase-B inhibitors and catechol-O–methyl transferase inhibitors.
Monoamine oxidase-B inhibitors may have disease modifying effect
Monoamine oxidase-B inhibitors and catechol-O–methyl transferase inhibitors rasigiline and selegiline inhibit enzymes involved in the breakdown of levodopa and dopamine and therefore prolong the effect of carbidopa/levodopa.
Monoamine oxidase-B inhibitors and catechol-O–methyl transferase inhibitors may increase the effects of levodopa, especially hallucinations, dyskinesia and nausea.
Anticholinergic medications are appropriate for early symptoms in cognitively normal patients whose primary symptom is tremor.
Dopamine agonists and particularly /levodopa are the most effective treatment for Parkinson’s.
Dopamine agonist pramipexole, ropinrole roll and rotigotine stimulate dopaminergic receptors in the CNS, which alleviate symptoms of Parkinson’ss disease.
The above dopamine agonists are less potent than levodopa but are frequently used because they are less likely to cause dyskinesia and tend to have a longer half-life.
More than 40% of patients with Parkinson’s disease treated with oral dopamine agonist such as ropinirole or pramipexole experience impulse control disorders of gambling, compulsive spending, abnormal sexual and eating behaviors, compulsive medication use, and hobbyism.
Levodopa is the most effective drug for motor symptom treatment.
Levodopa is given with , a peripheral decarboxylase inhibitor preventing levodopa conversion to dopamine in the peripheral circulation and liver.
Levodopa adverse effects include: nausea, vomiting, orthostatic hypotension, dyskinesia, hallucinations and psychosis.
Treating psychosis in Parkinson’s disease initially begins with weaning potentially contributing medications such as anticholinergics, dopamine agonist, amantadine, and sometimes levodopa.
Three agents for Parkinson associated psychoses are: pimavanserin, clozapine, and quetiapiine.
Commonly, insomnia, fatigue, and daytime sleepiness are disabling in PD.
Mild cognitive impairment can be present at Parkinson’s disease diagnosis or develop over time.
The probability of dementia in Parkinson’s disease is 46% at 10 years and among patients with a 20 year survival, 83% have dementia.
Hallucinations and paranoia may be seen, and generally appreciated in the setting of taking dopaminergic agents.
Early onset of hallucinations and delusions that appear or without taking medication, diagnosis of Lewy body disease is entertained.
Treatment:
Regular exercise, a healthy diet, quality sleep, and avoidance of adverse exposures reduces mortality and provides a foundation for management.
No pharmacologic therapy slows the progression of Parkinson’s disease.
Failure of pharmacological treatments is probably due to the fact that up to 75% of substantia nigra dopaminergic neurons have lost function even in the early stage of disease.
Symptom management is the aim of most treatments, and oral formulations of levodopa are the main treatment for motor symptoms.
The duration of effect after levodopa dose typically starts it several hours, and starts to shorten, on average, after four years.
Motor fluctuations are probably due too short half-life of the levodopa, inconsistent G.I. absorption, and progressive degeneration of dopaminergic neurons.
Common dose related side effects include dyskinesia, hallucinations, behavioral problems, orthostatic hypotension, and nausea.
Exogenous L-DOPA has been shown to be an effective treatment, however long-term use can result in increased dyskinesia and have deleterious effects on the survival of dopaminergic neurons.
Agonists Pramipexole and ropinirole although the primary alternatives to /levodopa, but are less efficacious and have more adverse effects including sedation, sleepiness, pathologic behaviors and a threefold risk of hallucinations.
Agonist therapy is utilized to reduce dyskinesia and motor fluctuations.
Dyskinesias are treated by decreasing the dopaminergic medications or adding amantadine.
30-50% of patients treated with a dopamine agonist or levodopa will have dopaminergic-related problems such as dyskinesias motor fluctuations and a wearing-off affect.
Most patients with Parkinson’ss disease have moderate to good dopaminergic medication response, but experience increased Parkinson disease symptoms when a medication dose wears off and experience dyskinesias overtime.
When the medication dose wears off it is referred to as off periods and off periods improve with the next medication dose and can occur within two years of initiating levodopa, and its prevalence increases over time.
Off periods are associated with disabilities and include motor and non-motor symptoms.
Levodopa slows the progression of Parkinson’s disease.
Rasagiline (Azilect)is a monoamine oxidase type B inhibitor approved for treating Parkinson’s disease.
Attenuation of Disease Progression with Azilect Given Once Daily (ADAGIO) trial of 1176 untreated Parkinson’s s disease patients randomly assigned to receive rasagiline 1 or 2 mg/day or placebo in an early treatment or late treatment groups: early treatment with 1 mg provided benefits consistent with possible disease modifying effect, but early treatment with 2 mg per day did not.
Medication therapy related complications develop in the majority of patients and include: dyskinesia, periods of loss of benefit from the medication.
Over time many symptoms such as gate, balance, speech, swallowing, or cognition may become progressively resistant to -levodopa and other drug therapies.
Levodopa is the most potent anti-Parkinson’s medication, and virtually all patients will be treated with it, at some point.
Levodopa is the immediate precursor of dopamine, which can cross the blood brain barrier.
Among patients with early Parkinson’s disease evaluated over the course of 80 weeks, treatment with levodopa in combination with carbidopa had no disease modifying effect (Verschuur CVM).
Lixisentide, a glucagon-like peptide 1 receptor agonist trial in early Parkinson’s disease resulted in less progression of motor disability, than placebo at 12 months.
Deep brain stimulation of bilateral subthalamic nuclei and globus pallidus internus efficacious for motor symptoms of PD.
Deep brain stimulation can reduce drug induced dyskinesias.
Deep brain stimulation involving surgical placement of leads transcranially in the subthalamic nucleus of the globus pallidus internal can reduce medication refractory tremor.
Deep brain stimulation outcomes are less good with adults older than 75 years, who have cognitive impairment, and who have been unresponsive to level dopa symptom relief.
Focused ultrasound subthalamotomy in one hemisphere improves motor features importance disease in selected patients with asymmetric signs.
Focused ultrasound to the thalamus can improve tremor, but is only helpful on one side of the body.
Typically levo-dopa-response of symptoms, tremor, on-off fluctuations from medications, and dyskinesia are most likely to improve with DBS.
Impairment of gait, balance, and speech are not improved by DBS and in some cases it may exacerbate the problem.
DBS is considered for Parkinson’s disease only after adequate trials of multiple medications have been unsuccessful.
Creatine monohydrate treatment compared with placebo does not improve clinical outcomes and this drug use is not supported by research (NINDS).
Midlife exercise is associated with his significant reduced later risk of Parkinson’s disease.
Regular aerobic type exercise leads to fitness and compelling evidence for slowing Parkinson disease progression (Ahlskog JE).
Dancing helps slow the progression of motor and nonmotor symptoms and improves quality of life for patients with Parkinson’s disease (PD)

One reply on “Parkinson’s disease”
[…] pramipexole, bromocriptine, ropinirole, pergolide and cabergoline and utilized for Parkinson’s disease, restless leg syndrome and hyperprolatinemic […]