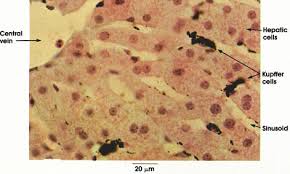
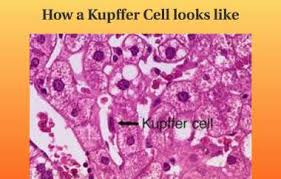
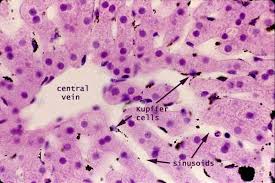
Kupffer cells, also known as stellate macrophages are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls.
Kupffer cells comprise the largest population of tissue-resident macrophages in the body.
Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells.
The Kupffer cells are first immune cells in the liver.
Changes to Kupffer cell function can be connected to various liver diseases: alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis.
Kupffer cells form part of the mononuclear phagocyte system.
Proinflammatory cytokines stimulate Kupffer cells produce IL-6 in the liver and present it to the hepatocytes.
Kupffer cells can be found attached to sinusoidal endothelial cells in both the centrilobular and periportal regions of the hepatic lobules.
Kupffer cell function and structures are depend on their location.
Periportal Kupffer cells tend to be larger and have more lysosomal enzyme and phagocytic activity, whereas centrilobular Kupffer cells create more superoxide radicals.
Kupffer cells are amoeboid in character, with surface features including microvilli, pseudopodia and lamellipodia, which project in every direction.
The microvilli and pseudopodia play a role in the endocytosis of particles.
The Kupffer nucleus is indented and ovoid, and can be lobulated.
Cytoplasmic elements in Kupffer cells include ribosomes, Golgi complexes, centrioles, microtubules, microfilaments, rough endoplasmic reticulum, a nuclear envelope, and annulate lamellae,
Cytoplasmic elements in Kupffer cells demonstrate peroxidase activity.
Kupffer cells express a scavenger receptor involved in recognizing and binding the lipid A domain of lipopolysaccharide (LPS) and lipoteichoic acid.
Lipopolysaccharide (LPS) is a bacterial endotoxin which is found in the cell wall gram-negative bacteria, and lipoteichoic acid is present in gram-positive bacteria, therefor Kupffer cells play a critical role in initiating and mediating immune responses to bacterial infection of the liver.
Under normal conditions, these Kupffer cell populations are long-lived and self-renewing.
Monocytes derived from hematopoietic stem cells in the bone marrow and transported through blood circulation to the liver can also fully differentiate into true Kupffer cells, and are capable of self-renewal once a population is established.
Kupffer cells are regulated by numerous growth factors, with macrophage colony-stimulating factor (CSF1) playing a key role.
Cytokines involved in type 2 inflammation, such as IL-4, may also stimulate Kupffer cell proliferation.
Hepatic Kupffer cell populations are tightly maintained.
There is a high rate of turnover, with the average lifespan of a Kupffer cell estimated at 3.8 days.
The primary function of the Kupffer cell is to remove foreign debris and particles that have come from the hepatic portal system when passing through the liver.
It is thought the Kupffer cells to take in large particles by phagocytosis and smaller particles via pinocytosis.
Kupffer cells are integral in the innate responses of the immune system, being important for host defense and play a role in the metabolism of many different compounds including, lipids, protein complexes and small particles.
Kupffer cells are useful in removing apoptotic cells from circulation.
The amount of Kupffer cells in the liver is held constant.
Kupffer cells have a proliferative capacity, allowing for cell populations to replenish themselves.
Monocyte-derived macrophages that have no proliferative potential.
Old or defective Kupffercells are removed through apoptosis, as well as through being phagocytized by neighbouring Kupffer cells.
Kupffer cells function varies, dependent on their location in the liver lobules.
Kupffer cells in the periportal zone are directly exposed to bloodflow, and express greater lysosomal activity to more efficiently process incoming foreign substances.
In Kupffer cells in the centrilobular zone experience less perfusion, and are equipped with greater stores of superoxide to combat deeply-penetrating injuries and infections.
Kupffer cells can produce inflammatory cytokines, TNF-alpha, oxygen radicals, IL-10 and proteases in response to infection or injury, which in excess, are linked to the development of liver injury.
Kupffer cells clear bacteria, are responsible for recycle hemoglobin by destroying senescent red blood cells through phagocytic action.
The globin chains are re-used, while the iron-containing portion, heme, is further broken down into iron, which is re-used, and bilirubin, which is conjugated to glucuronic acid within hepatocytes and secreted into the bile.
Complement receptor of the immunoglobulin (CRIg) in Kupffer cells is a critical component of the innate immune system to clear complement system-coated pathogens.
Kupffer cells play a role in the pathogenesis of a damaged liver in response to sepsis, by activating and release both IL-1 and TNF-alpha.
These released substances activate leukocytes and sinusoidal endothelial cells to express ICAM-1, resulting in tissue damage to the endothelium because of proteases, oxygen radicals, prostanoids and other substances from leukocytes.
Kupffer cell activation contributes to both chronic and acute alcoholic liver disease in response to ethanol-induced liver injury.
Chronic alcoholism and liver injury are related by a two-hit system: the first hit is direct toxicity of ethanol and its metabolic byproducts, the second hit is indirect, mediated by increased uptake of lipopolysaccharide, as endotoxin, from the intestine.
Ethanol increases permeability of the intestinal epithelium, resulting in endotoxin produced by the intestinal flora leaking from the intestinal lumen into the liver via the portal vein.
The presence of endotoxin induces a strong polarization of Kupffer cells, and a large amount of reactive oxygen species, pro-inflammatory cytokines and chemokines are produced by the activated Kupffer cells which lead to liver injury.
There is an endotoxin-mediated activation of the Toll-like receptor 4 (TLR4) and CD14, receptors on the Kupffer cell that internalize endotoxin, which activates the transcription of pro-inflammatory cytokines and tumor necrosis factor-alpha (TNFα), with concurrent production of superoxides.
Cytokines and superoxides cause inflammation and oxidizing damage respectively, while TNFα triggers the stellate cells in the liver to initiate collagen synthesis.
These processes result in fibrosis, or scarring of the liver.
Fibrosis will eventually cause cirrhosis, a loss of function of the liver due to extensive scarring.
